Copyright
©The Author(s) 2021.
World J Stem Cells. Jun 26, 2021; 13(6): 485-502
Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.485
Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.485
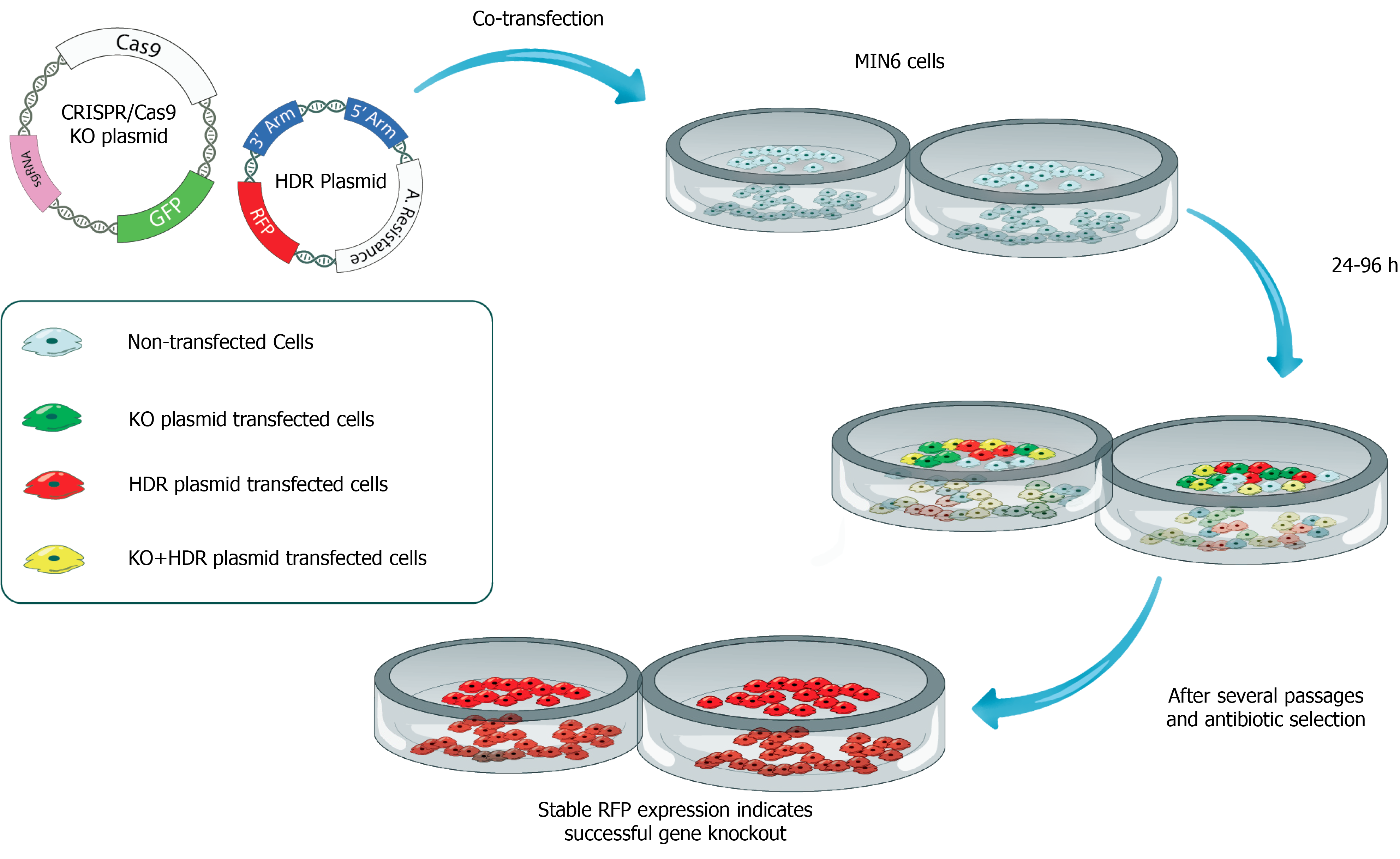
Figure 3 Production of CRISPR/Cas9-mediated insulin-deficient pancreatic beta cell line for testing the therapeutic efficacy of insulin gene therapy vectors.
In this scenario, the CRISPR/Cas9 knockout plasmid carries a Cas9 protein-encoding sequence, sgRNA, and green fluorescein protein as a reporter. Homology-directed repair (HDR) plasmid contains two genomic fragments of DNA complementary to insulin gene (3’ Arm and 5’ Arm) and red fluorescein protein-encoding DNA sequences. Both CRISPR/Cas9 knockout plasmid and HDR plasmid are transfected into a pancreatic beta-cell line such as MIN6 cells. After several passages and antibiotic selection, beta cells expressing red fluorescent protein remain alive suggesting successful knockout (KO) of the insulin (INS) gene. INS KO cells can be cloned by limited dilution and further expanded in cell culture. INS KO cells can be used in in vitro complementation assays as well as transplanted under the kidney capsule of diabetic rats to assess the therapeutic efficacy of gene therapy vectors. KO: Knockout; HDR: Homology-directed repair.
- Citation: Eksi YE, Sanlioglu AD, Akkaya B, Ozturk BE, Sanlioglu S. Genome engineering and disease modeling via programmable nucleases for insulin gene therapy: Promises of CRISPR/Cas9 technology . World J Stem Cells 2021; 13(6): 485-502
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/485.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.485