Copyright
©The Author(s) 2021.
World J Stem Cells. May 26, 2021; 13(5): 416-438
Published online May 26, 2021. doi: 10.4252/wjsc.v13.i5.416
Published online May 26, 2021. doi: 10.4252/wjsc.v13.i5.416
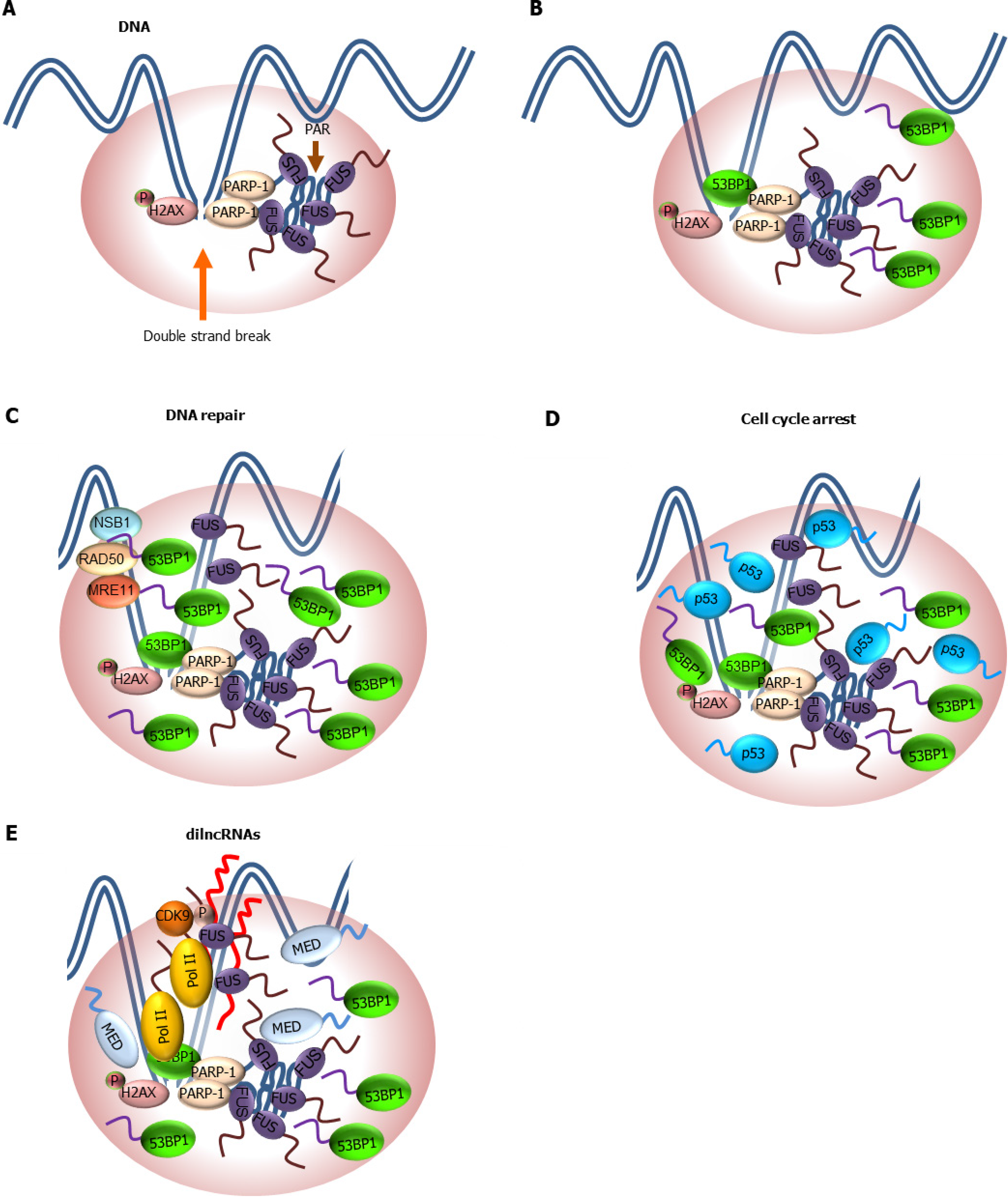
Figure 4 Role of low complexity domain-driven condensate formation in DNA damage response and DNA repair.
A: Double strand break (DSB) triggers phosphorylation of histone variant H2AX (γ-H2AX) and poly-ADP-ribosylation (PARylation) by PARP-1. PARylation facilitates the recruitment of fused in sarcoma (FUS) to DSB site through its RGG domain and formation of FUS condensates driven by its prion-like low complexity domain (LCD); B: γ-H2AX and FUS condensates at DSBs promote the incorporation of a critical downstream effector of DSB repair factor, p53-binding protein 1 (53BP1). However, the Tudor domain but not its disordered region of 53BP1 is required for 53BP1 phase separation; C: Formation of 53BP1/FUS condensates facilitate the recruitment of downstream repair machinery such as meiotic recombination 11 homolog. RAD50 homolog, double strand break repair protein, and Nijmegen breakage syndrome 1 involved in homologous recombination repair of DSBs; D: p53 is incorporated into 53BP1 condensates and activate a p53-dependenent gene expression that results in cell cycle arrest. Disruption of 53BP1 condensates blunts p53-dependent response to DNA damage; E: Assembly of Pol II, mediator and cyclin dependent kinase 9/positive transcription elongation factor b at DSBs leads to transcription of dilncRNAs. dilncRNAs facilitates molecular crowding and phase separation of 53BP1 and other repair factors. It is likely that a network of LCD-mediated protein-protein and protein-nucleic acid interactions drives the formation of repair condensates at DSBs. 53BP1: p53-binding protein 1; dilncRNA: Damage-induced long non-coding RNA; DSB: Double strand break; FUS: Fused in sarcoma; H2AX: Histone variant 2AX; MRE11: Meiotic recombination 11 homolog; NBS1: Nijmegen breakage syndrome 1; PARP-1: Poly-ADP-ribose polymerase-1; RAD50: RAD50 homolog, double strand break repair protein.
- Citation: Vodnala M, Choi EB, Fong YW. Low complexity domains, condensates, and stem cell pluripotency. World J Stem Cells 2021; 13(5): 416-438
- URL: https://www.wjgnet.com/1948-0210/full/v13/i5/416.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i5.416