Copyright
©The Author(s) 2021.
World J Stem Cells. Feb 26, 2021; 13(2): 177-192
Published online Feb 26, 2021. doi: 10.4252/wjSC.v13.i2.177
Published online Feb 26, 2021. doi: 10.4252/wjSC.v13.i2.177
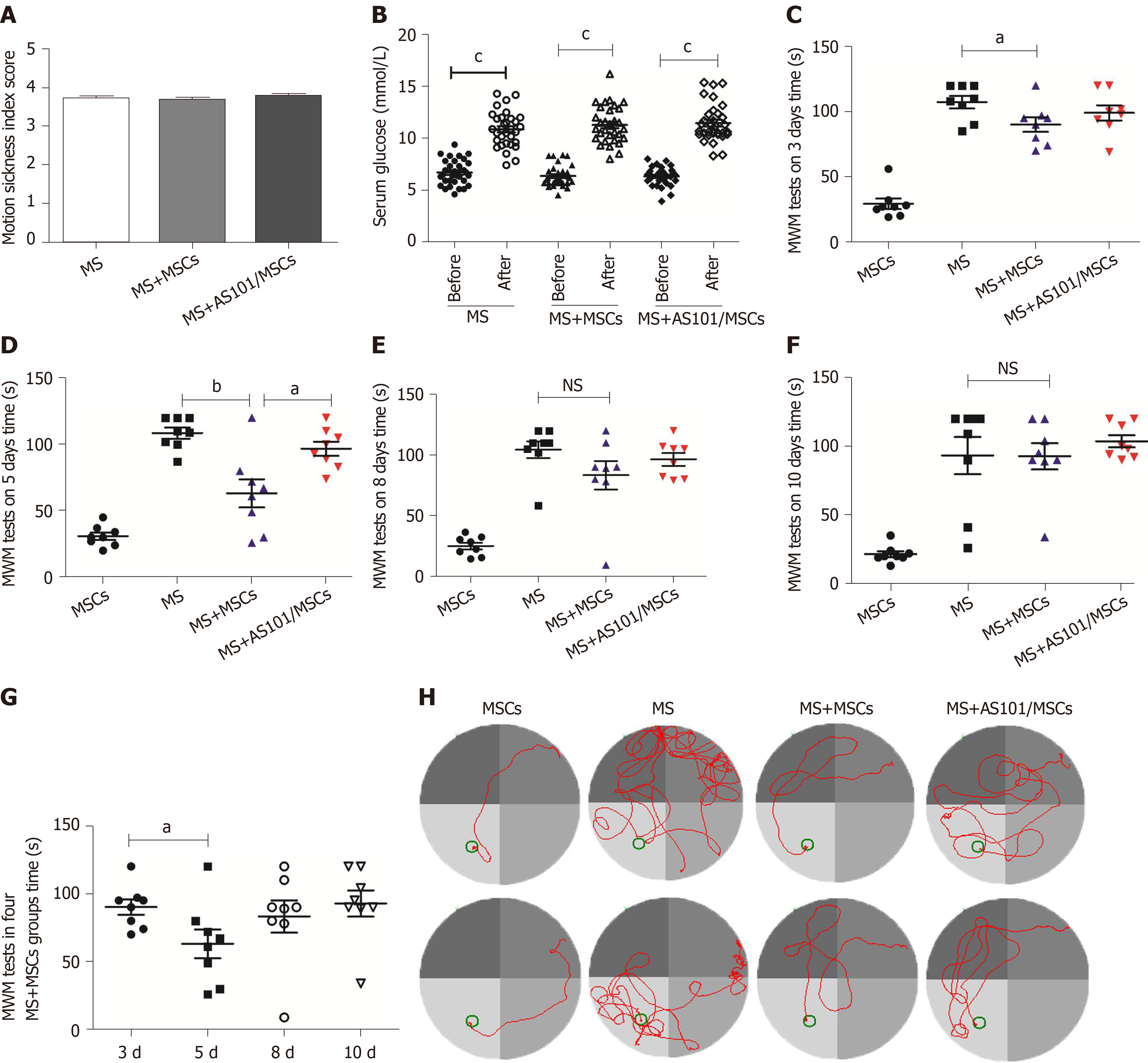
Figure 3 Motion sickness model evaluation and effects of umbilical cord-derived mesenchymal stem cells treatment on performance of mice in Morris water maze tests.
A: Motion sickness (MS) index score reflecting the degree of MS symptoms in mice; B: Serum glucose levels in the three groups before and after exposure to rotation (cP < 0.001); C-F: Time taken for the mice to reach the platform after different treatments. The time of the MS + mesenchymal stem cells (MS + MSCs) group was faster than that of the MS group at 3 d and 5 d (aP < 0.05; bP < 0.01); G: The 5-day group had the shortest time among the four MS + MSCs subgroups (aP < 0.05); H: Swimming trajectories in the MSCs, MS, MS + MSCs, and MS + AS101/MSCs groups on day 5; the platform is shown as a small circle. In addition to the MSCs group, the path of mice in the MS + MSCs group was clearer. MWM: Morris water maze; MS: Motion sickness; MSCs: Mesenchymal stem cells.
- Citation: Zhu HS, Li D, Li C, Huang JX, Chen SS, Li LB, Shi Q, Ju XL. Prior transfusion of umbilical cord mesenchymal stem cells can effectively alleviate symptoms of motion sickness in mice through interleukin 10 secretion. World J Stem Cells 2021; 13(2): 177-192
- URL: https://www.wjgnet.com/1948-0210/full/v13/i2/177.htm
- DOI: https://dx.doi.org/10.4252/wjSC.v13.i2.177