Copyright
©The Author(s) 2020.
World J Stem Cells. Feb 26, 2020; 12(2): 139-151
Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.139
Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.139
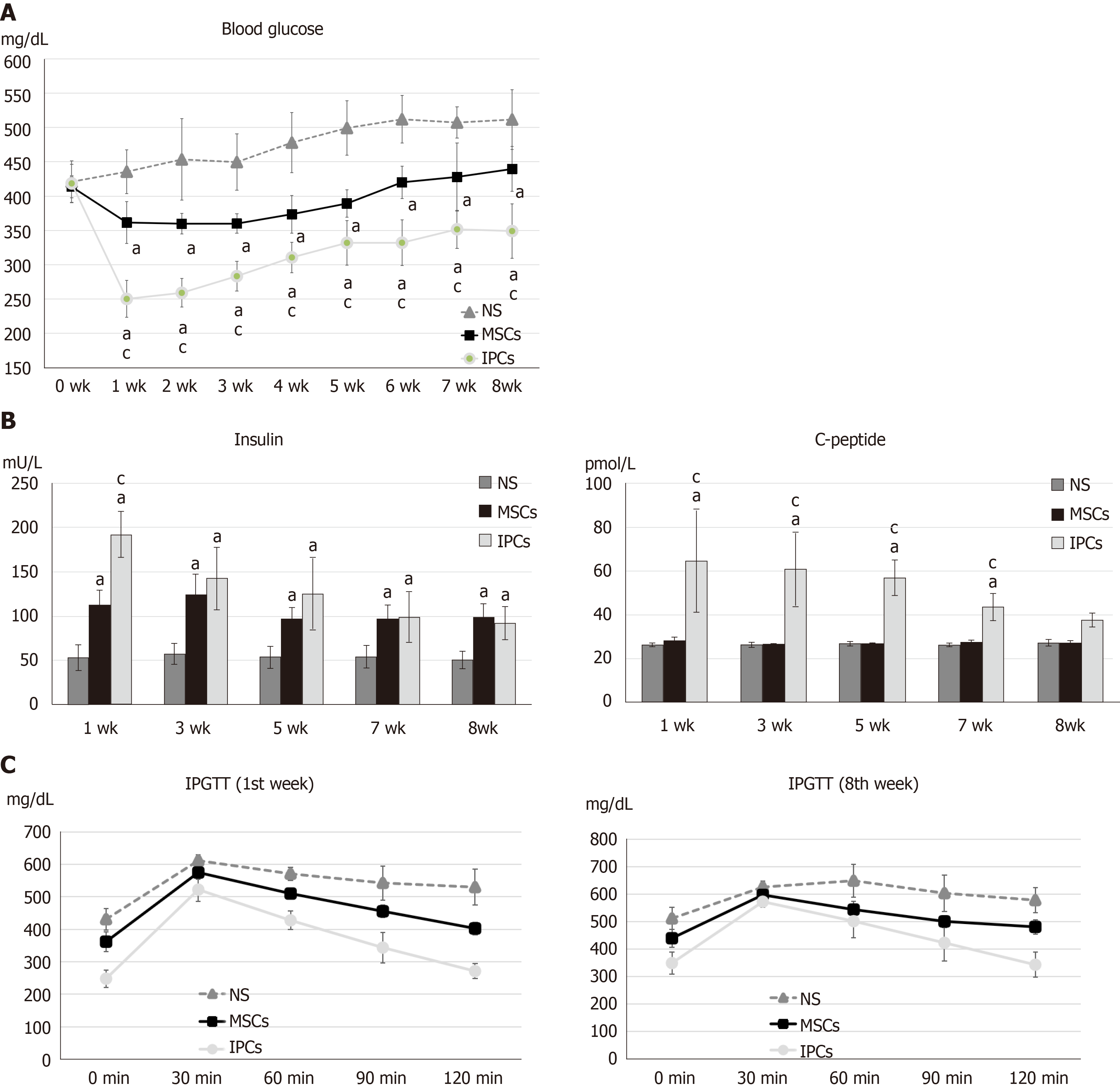
Figure 3 Comparison of differences in blood glucose, serum insulin, serum C-peptide, and intraperitoneal glucose tolerance test results between streptozotocin-induced diabetic rats treated with undifferentiated human Wharton's jelly mesenchymal stem cells and insulin-producing cells.
A: aP < 0.05, compared to the normal saline (NS) treatment group, the rats in the two treatment groups had significantly decreased blood glucose levels; cP < 0.05, blood glucose levels in rats in the insulin-producing cell (IPC) group were significantly lower than in rats from the undifferentiated human Wharton’s jelly mesenchymal stem cell (hWJ-MSC) group; B: aP < 0.05, compared to the NS treatment group, the rats in other two treatment groups had significantly higher serum insulin levels; cP < 0.05, serum insulin levels in rats from the IPC group were significantly higher than those in rats from the undifferentiated hWJ-MSC group; aP < 0.05, compared to the NS treatment group, rats in the IPC treatment group had significantly higher serum C-peptide levels; cP < 0.05, serum C-peptide level blood glucose level of rats from the IPC group was significantly higher than those in rats from the undifferentiated hWJ-MSC group; C: IPC and MSC treatment led to better improvement in the intraperitoneal glucose tolerance test (IPGTT) result than NS treatment in both the first and eighth weeks.
- Citation: Hsiao CY, Chen TH, Huang BS, Chen PH, Su CH, Shyu JF, Tsai PJ. Comparison between the therapeutic effects of differentiated and undifferentiated Wharton's jelly mesenchymal stem cells in rats with streptozotocin-induced diabetes. World J Stem Cells 2020; 12(2): 139-151
- URL: https://www.wjgnet.com/1948-0210/full/v12/i2/139.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i2.139