Copyright
©The Author(s) 2020.
World J Stem Cells. Jan 26, 2020; 12(1): 35-54
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.35
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.35
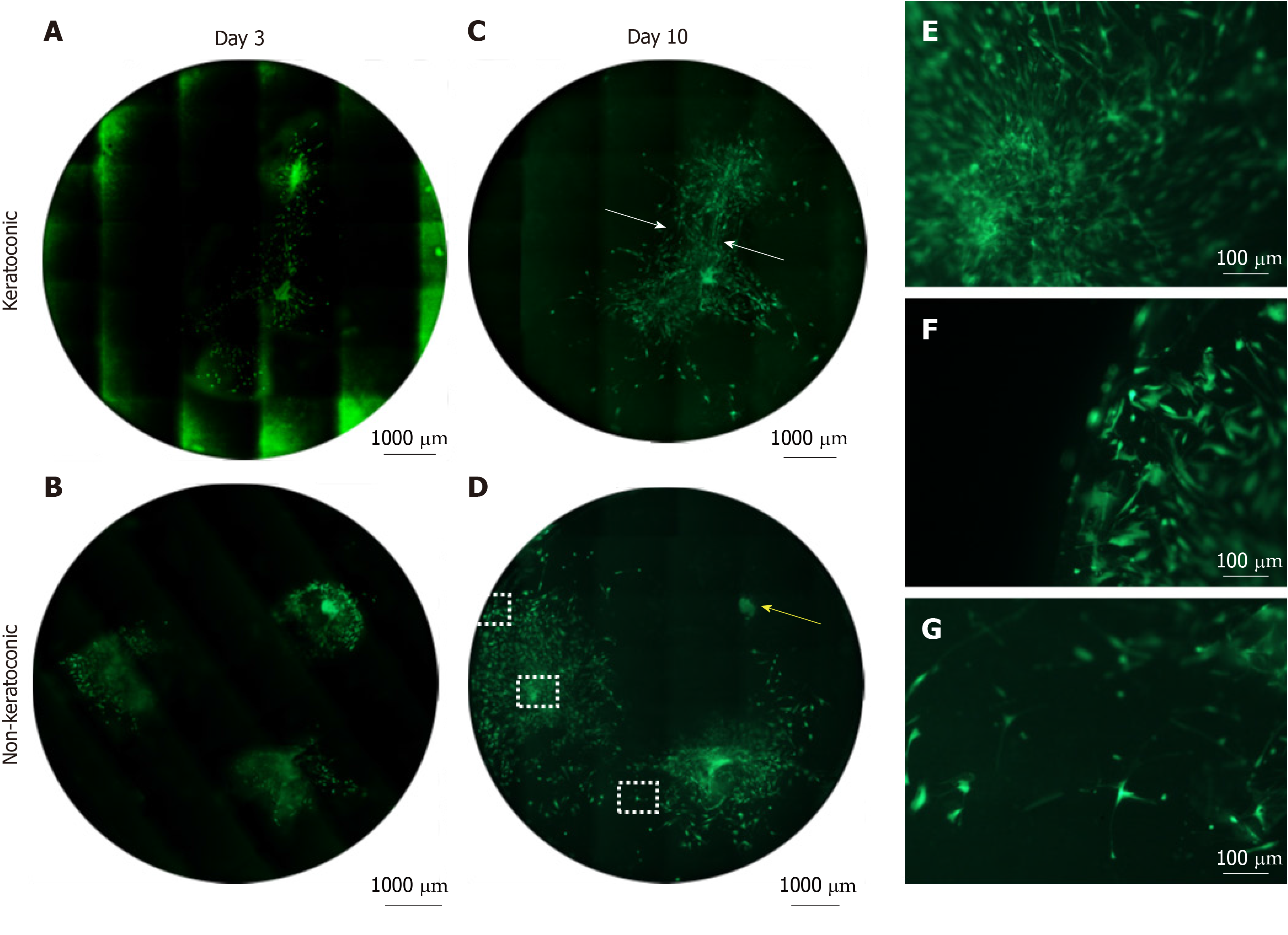
Figure 5 Cell viability of spheres and sphere-derived cells within implanted full-thickness corneal tissue.
A-D: Decellularised full-thickness central corneal stromal tissues (corneal buttons) implanted with three spheres each and assessed for cell viability with Calcein-AM (green) were imaged at 50 × magnification with a fluorescence microscope and montaged to maintain detail of individual cells throughout the entire corneal button. Each circle represents the corneal button edge. Three days after implantation (day 3), spheres in keratoconic (A) and non-keratoconic (decompensated: normal anterior cornea with failed endothelium) (B) matrices are viable and have viable cells radiating from the sphere. At day 10, spheres in keratoconic (C) and non-keratoconic (D) matrices remain viable, and the viable cells have migrated further outward from the centre of the sphere. An exception to this was one sphere within non-keratoconic tissue, which showed reduced cell migration at day 10 compared with day 3 (yellow arrow, D); E-G: Panels E-G are magnified areas from D indicated by dotted squares (E = central square, F = top square, G = lower square). When spheres are implanted close to each other, cells align between them as if forming “cellular bridges” (white arrows, C). Cells near the centre of the sphere orientate radially from the centre of the sphere (E) while cells near the tissue edge are aligned parallel with the edge (F). Cells distant from both the nearest sphere and the tissue edge appear to lose their alignment (G). Scale bar = 1000 µm for A-D and 100 µm for E-G. Bright green fluorescence at left edge of tiles in montage (A) is artefactual.
- Citation: Wadhwa H, Ismail S, McGhee JJ, Van der Werf B, Sherwin T. Sphere-forming corneal cells repopulate dystrophic keratoconic stroma: Implications for potential therapy. World J Stem Cells 2020; 12(1): 35-54
- URL: https://www.wjgnet.com/1948-0210/full/v12/i1/35.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i1.35