修回日期: 2016-10-18
接受日期: 2016-10-24
在线出版日期: 2016-12-28
本研究拟通过RNA干扰技术下调人类肝癌细胞(hepatocellular carcinoma, HCC)CD98基因的表达, 探讨CD98对肿瘤侵袭与转移的影响.
利用CD98 siRNAs瞬时转染人类HCC系(FHCC-98), 免疫印迹法证实癌细胞中CD98蛋白表达下调后, 通过细胞黏附实验、Transwell侵袭实验及划痕愈合实验, 检测CD98对HCC的黏附、侵袭和迁移能力的影响.
CD98 siRNAs瞬时转染FHCC-98细胞48 h后, 癌细胞CD98的蛋白水平显著下调, 干扰CD98蛋白表达水平后, 癌细胞黏附能力显著下降, Transwell实验证实与对照组相比, 癌细胞的侵袭能力显著下降, 划痕实验也证实干涉CD98后癌细胞的迁移能力显著下降.
CD98通过增强HCC的黏附及迁徙能力, 促进HCC的侵袭和转移, 为未来肝细胞癌治疗提供了可能的靶点.
核心提要: 本实验利用RNAi技术抑制FHCC-98肝癌细胞的CD98表达后, 癌细胞的黏附、侵袭和迁移能力明显下降, 证实CD98能够促进肝细胞癌的浸润和转移, 是肝癌发生及演进过程中的肿瘤促进因子.
引文著录: 贾国战, 翟玉龙, 周帅, 张波, 吴涛, 何显力, 乔庆. CD98对肝癌细胞侵袭和转移的影响. 世界华人消化杂志 2016; 24(36): 4788-4793
Revised: October 18, 2016
Accepted: October 24, 2016
Published online: December 28, 2016
To knock down CD98 expression in human hepatocellular carcinoma (HCC) cells by RNA interference (RNAi) to explore the role of CD98 in HCC progression.
Down-regulation of CD98 expression in human HCC cell line (FHCC-98) was performed by RNAi and verified by Western blot analysis. The role of CD98 in the invasion and metastasis of FHCC-98 cells was investigated using cell adhesion assay, transwell invasion assay, and monolayer wound healing assay.
After transient transfection with siRNA targeting CD98 for 48 h, the protein expression of CD98 in human HCC cells was significantly knocked down. Moreover, the numbers of attached cells and invaded cells, and wound closure rate were significantly decreased compared to cells transfected with the negative control siRNA.
CD98 may promote tumor invasion and metastasis in HCC via enhancement of the abilities of tumor cells to adhere, invade and migrate. CD98 may be a novel therapeutic target for the treatment of HCC.
- Citation: Jia GZ, Zhai YL, Zhou S, Zhang B, Wu T, He XL, Qiao Q. Role of CD98 in invasion and metastasis of hepatocellular carcinoma cells. Shijie Huaren Xiaohua Zazhi 2016; 24(36): 4788-4793
- URL: https://www.wjgnet.com/1009-3079/full/v24/i36/4788.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i36.4788
原发性肝细胞癌(hepatocellular carcinoma, HCC)是严重危害人类健康的恶性肿瘤之一, 在全世界范畴, HCC发病率位居常见癌症的第5位、癌症相关死亡率的第3位, 其临床主要表现为肿瘤的快速进展率及高复发率, 转移作为肝癌的最主要的恶性表型, 是导致HCC高致死率的主要因素[1,2]. CD98是一种Ⅱ型跨膜糖蛋白, 由一条重链(CD98hc, SLC3A2)和一条轻链(包括LAT-1等)通过二硫键共价连接而成的异源二聚体[3,4], 除血小板外, 可以表达于所有细胞的表面, 其基因序列和蛋白结构在不同物种进化过程中高度保守. CD98参与细胞黏附及细胞外基质合成过程, 并参与多种细胞信号转导通路, 与肿瘤的发生和转移密切相关[5-8], 但是有关CD98对HCC的作用机制研究鲜有报道. 因此, 本研究拟通过RNA干扰技术, 下调人类HCC系(FHCC-98)中CD98的蛋白水平, 结合细胞黏附、侵袭和迁移实验检测CD98对HCC转移能力的影响, 以期为未来肝癌靶向治疗提供新的靶点.
人类HCC系FHCC-98由第四军医大学唐都医院肿瘤科构建[9,10], 细胞培养于含100 mL/L胎牛血清的完全RPMI 1640培液中, 置于37 ℃、50 mL/L CO2饱和湿度的培养箱中培养. 细胞培养液RPMI 1640购自美国GIBCO BRL公司; 小牛血清为杭州四季青公司产品; Matrigel购自美国BD生物科学公司; BSA及结晶紫购自美国Sigma公司; 鼠抗人单克隆抗体CD98及Tublin购自美国Santa cruz公司; CD98 siRNAs设计参照文献[11,12], 由上海生工生物工程公司合成; LipofectamineTM 2000试剂盒购自美国Invitrogen公司.
1.2.1 细胞转染及筛选: 接种FHCC-98细胞于6孔板, 待细胞密度达到60%, 加入适当浓度CD98 siRNAs和LipofectamineTM 2000, 继续培养48 h后, 备用. 实验设立CD98 RNAi组及无关干涉片段(control siRNA)对照组; 实验前对CD98 siRNAs和LipofectamineTM 2000浓度进行预先优化, 利用美国Invitrogen公司的BLOCK-iTTM荧光寡聚物作为转染效率的评估指标.
1.2.2 免疫印迹实验: 留取各组细胞, 经含1% PMSF的RIPA裂解液裂解后, 利用BCA蛋白定量试剂盒对待测样品中总蛋白含量进行定量分析. 每组取10 μg蛋白, 加入上样缓冲液后煮沸、变性, 进行10%SDS-PAGE电泳; 将蛋白电转至PVDF膜上, 5%脱脂奶粉封闭液中室温封闭2 h后, 分别加入CD98抗体(1:800)、Tublin抗体(1:2000)4 ℃孵育过夜; TBST洗膜4次后, 加入二抗(1:5000)室温孵育2 h; TBST洗膜4次, 加入ECL试剂显影、拍照, 利用蛋白灰度分析软件进行定量.
1.2.3 细胞黏附实验: 用Matrigel(5 μg/mL)按50 μL/孔包被96孔细胞培养板, 4 ℃过夜后, 20 mL/L BSA的PBS室温封闭30 min, PBS洗涤后备用. 取对数生长期的各组细胞按2×104细胞/孔, 接种于培养板上, 培养30-60 min后弃去培养液和未黏附细胞, 每孔加入50 μL结晶紫, 室温放置10 min; 流水冲洗去除多余染料、空气干燥后, 加入100 μL细胞裂解液, 室温放置20 min, 酶标仪检测540 nm波长处的吸光值(A540).
1.2.4 Transwell细胞侵袭实验: 将Transwell小室放入24孔板中, 将稀释的Matrigel(5 μg/mL)加入小室内微孔滤膜上4 ℃过夜, 向小室内加入1×105个细胞、小室外加入RPMI 1640完全培养液作为趋化剂, 培养48 h. 取出Transwell小室, 弃培养液, 用棉签擦去微孔滤膜上层的细胞, 甲醛固定后, 进行苏木素-伊红(HE)染色, 中性树胶封片. 在400×光学显微镜下计数穿过凝胶和微孔的细胞数, 随机计数中间和四周共5个视野, 每个视野计数细胞3次, 求平均值, 实验重复3次.
1.2.5 细胞迁移实验: 将2×106细胞/孔均匀接种于6孔板中, 置于培养箱孵育至细胞贴壁并形成均匀单层后, 进行划痕; 无血清RPMI 1640培养液轻洗细胞3次、去除游离细胞, 倒置显微镜下拍照; 继续培养24 h后, 再次拍照; 每次拍照均利用Image J软件测量划痕宽度. 每组实验重复3次, 计算划痕修复率. 划痕修复率 = (0 h的划痕宽度-24 h的划痕宽度)/0 h的划痕宽度×100%.
统计学处理 利用SPSS18软件进行Student-t检验, 每组实验均至少重复3次, 结果以mean±SD表示, 以P<0.05为差异具有统计学意义.
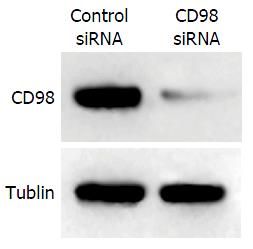
利用人工合成的CD98 siRNAs转染FHCC-98细胞48 h后, Western blot法检测CD98蛋白表达的情况, 结果表明, 与转染无关干涉片段的FHCC-98细胞相比, CD98 siRNAs能够明显抑制CD98蛋白表达(P<0.01, 图1).
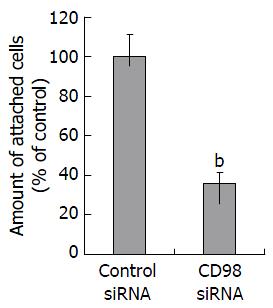
细胞黏附实验结果显示, FHCC-98细胞瞬时转染CD98 siRNAs 48 h后, 细胞黏附率较对照组明显降低(P<0.01, 图2).
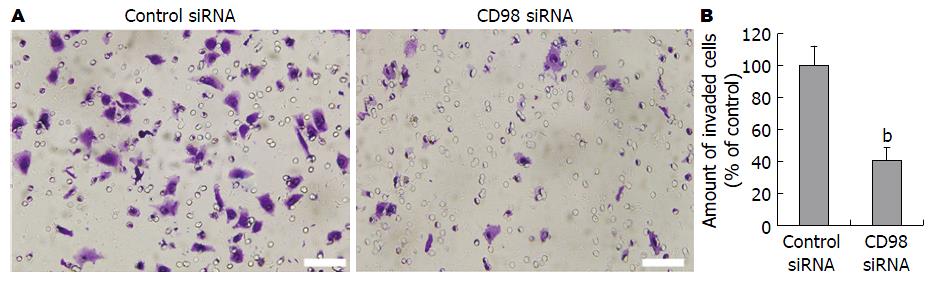
本实验利用铺有Matrigel的Transwell小室检测CD98对HCC侵袭能力的影响, 结果显示: 在培养24 h之后, 转染CD98 siRNAs的FHCC-98细胞黏附率较对照组降低了56.7%±5.2%(P<0.01, 图3).
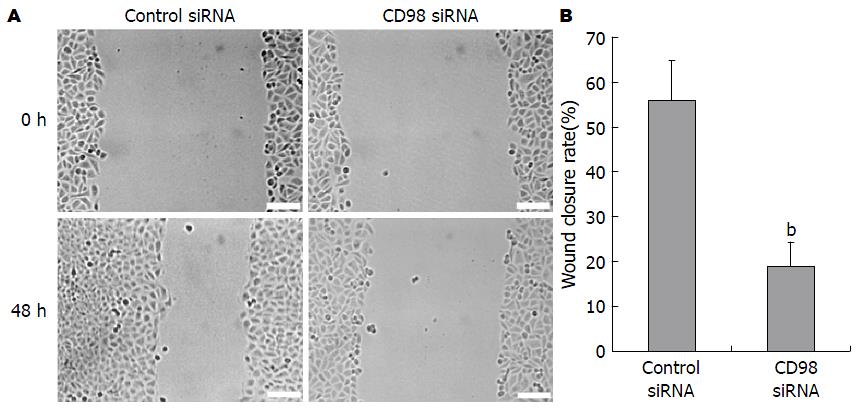
为检测CD98对HCC迁移能力的影响, 本课题组利用RNAi瞬时转染FHCC-98细胞48 h后、CD98蛋白表达下调, 细胞划痕实验结果显示: 与无关对照组的划痕修复率56.0%±8.9%相比, 转染CD98 siRNAs的FHCC-98细胞的划痕修复率仅为19.3%±5.1%(P<0.01, 图4). 上述结果提示CD98蛋白对HCC迁移能力的调节可能具有重要的调节作用.
侵袭与转移是HCC最主要的恶性表型, 促进了肝癌的演进过程, 与患者的不良预后显著相关[13,14], 在本实验研究中, 我们利用RNAi技术抑制FHCC-98 HCC的CD98表达后, 癌细胞的黏附、侵袭和迁移能力明显下降, 该结果提示CD98分子能够促进HCC的侵袭与转移.
肿瘤的发生及演进过程是肿瘤细胞与其微环境相互作用的过程[15,16], 细胞外基质蛋白和黏附分子均参与此过程并发挥重要作用[17,18], CD98通过其重链CD98hc与整合素相互作用, 活化Akt和Rac GTP酶[19], CD98通过其轻链参与氨基酸转运[20,21]. 目前认为CD98能够与β1整合素结合, 通过β1整合素调节整合素信号途径, 进而调控细胞增殖、存活、迁徙、上皮细胞黏附性及极向[22].
已有文献报道[23-29], CD98高表达于人类恶性黑色素瘤、喉部鳞状细胞癌、肺腺癌、乳腺癌及肾细胞癌, 但是有关CD98在HCC中的研究鲜有报道. Namikawa等[30]发现CD98轻链LAT-1与重链CD98hc/4F2h在88例人类HCC组织中的阳性率分别为61%和77%. 已有文献证实, LAT1的表达量是HCC患者的独立预后因素[31], LAT1高表达的患者与预后不良显著相关[32], 而尚无明确研究证实CD98hc表达与患者预后之间的关系, 在我们所选用的HCC系FHCC-98也有较高的CD98表达, 利用RNAi技术抑制FHCC-98 HCC的CD98表达可显著降低癌细胞的黏附、侵袭和迁移能力, 也支持上述文献结果.
总之, 本研究结果证实CD98能够促进HCC的浸润和转移, 是肝癌发生及演进过程中的肿瘤促进因子, CD98可以作为抑制HCC侵袭和转移的潜在靶点, 开发靶向CD98的药物可能成为治疗肝癌的新策略.
CD98参与细胞黏附及细胞外基质合成过程, 并参与多种细胞信号转导通路, 与肿瘤的发生和转移密切相关.
近年来恶性肿瘤转移与侵袭等恶性行为受到广泛关注, 探究转移与侵袭相关重点分子已成为相关领域研究热点.
目前已有文献证实CD98在乳腺癌及其他恶性肿瘤生物学行为中起到关键作用, 但尚未见到CD98与肝癌恶性表型相关的研究报道.
本文运用多种细胞生物学手段, 对CD98在肝癌细胞(hepatocellular carcinoma, HCC)侵袭和转移等生物学行为中的作用进行了探究, 对HCC侵袭和转移等生物学行为进行了进一步的研究.
本文通过对CD98在HCC侵袭和转移等生物学行为中的探索, 系统研究了CD98对HCC的侵袭和转移的调节作用, 为治疗肝癌提供了潜在的靶点.
方哲平, 主任医师, 浙江省台州医院肝胆外科; 沈东炎, 副教授, 厦门大学附属第一医院中心实验室; 史颖弘, 副教授, 副主任医师, 复旦大学附属中山医院肝癌研究所; 朱小三, 主治医师, 厦门大学附属成功医院消化内科
本文报道了CD98对HCC侵袭和转移的调节作用, 国内外相关研究甚少, 具有较好的创新性, 同时为未来HCC治疗提供了潜在的靶点.
手稿来源: 自由投稿
学科分类: 胃肠病学和肝病学
手稿来源地: 陕西省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 闫晋利 电编:胡珊
| 1. | Jiang G, Zhang L, Zhu Q, Bai D, Zhang C, Wang X. CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2016;35:38. [PubMed] [DOI] |
| 2. | Breuer K, Matterne U, Diepgen TL, Fartasch M, Gieler U, Kupfer J, Lob-Corzilius T, Ring J, Scheewe S, Scheidt R. Predictors of benefit from an atopic dermatitis education programme. Pediatr Allergy Immunol. 2014;25:489-495. [PubMed] [DOI] |
| 3. | Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373-1382. [PubMed] [DOI] |
| 4. | Nguyen HT, Merlin D. Homeostatic and innate immune responses: role of the transmembrane glycoprotein CD98. Cell Mol Life Sci. 2012;69:3015-3026. [PubMed] [DOI] |
| 5. | Kaira K, Sunose Y, Oriuchi N, Kanai Y, Takeyoshi I. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat Dis Int. 2014;13:654-657. [PubMed] [DOI] |
| 6. | Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D'Amato M, Sitaraman SV. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733-1747. [PubMed] [DOI] |
| 7. | Rietbergen MM, Martens-de Kemp SR, Bloemena E, Witte BI, Brink A, Baatenburg de Jong RJ, Leemans CR, Braakhuis BJ, Brakenhoff RH. Cancer stem cell enrichment marker CD98: a prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Eur J Cancer. 2014;50:765-773. [PubMed] [DOI] |
| 8. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473-3481. [PubMed] [DOI] |
| 9. | Lou CY, Feng YM, Qian AR, Li Y, Tang H, Shang P, Chen ZN. Establishment and characterization of human hepatocellular carcinoma cell line FHCC-98. World J Gastroenterol. 2004;10:1462-1465. [PubMed] [DOI] |
| 10. | Ma T, Wang Z, Yang Z, Chen J. Cluster of differentiation 147 is a key molecule during hepatocellular carcinoma cell-hepatic stellate cell cross-talk in the rat liver. Mol Med Rep. 2015;12:111-118. [PubMed] [DOI] |
| 11. | Laroui H, Geem D, Xiao B, Viennois E, Rakhya P, Denning T, Merlin D. Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther. 2014;22:69-80. [PubMed] [DOI] |
| 12. | Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, Zhang Z, Baker MT, Zhang B, Gewirtz AT. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146:1289-300.e1-19. [PubMed] [DOI] |
| 13. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [PubMed] [DOI] |
| 14. | Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92:1515-1542. [PubMed] [DOI] |
| 15. | Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40:1733-1747. [PubMed] [DOI] |
| 16. | Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. [PubMed] [DOI] |
| 17. | Brower V. Adhesion Molecules, Stem Cells, and the Microenvironment in Acute Myeloid Leukemia. J Natl Cancer Inst. 2016;108:pii: djw113. [PubMed] [DOI] |
| 18. | Sero JE, Sailem HZ, Ardy RC, Almuttaqi H, Zhang T, Bakal C. Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. Mol Syst Biol. 2015;11:790. [PubMed] [DOI] |
| 19. | Rintoul RC, Buttery RC, Mackinnon AC, Wong WS, Mosher D, Haslett C, Sethi T. Cross-linking CD98 promotes integrin-like signaling and anchorage-independent growth. Mol Biol Cell. 2002;13:2841-2852. [PubMed] [DOI] |
| 20. | Cibrian D, Saiz ML, de la Fuente H, Sánchez-Díaz R, Moreno-Gonzalo O, Jorge I, Ferrarini A, Vázquez J, Punzón C, Fresno M. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat Immunol. 2016;17:985-996. [PubMed] [DOI] |
| 21. | Papin-Michault C, Bonnetaud C, Dufour M, Almairac F, Coutts M, Patouraux S, Virolle T, Darcourt J, Burel-Vandenbos F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS One. 2016;11:e0157139. [PubMed] [DOI] |
| 22. | Lee KJ, Yoo YH, Kim MS, Yadav BK, Kim Y, Lim D, Hwangbo C, Moon KW, Kim D, Jeoung D. CD99 inhibits CD98-mediated β1 integrin signaling through SHP2-mediated FAK dephosphorylation. Exp Cell Res. 2015;336:211-222. [PubMed] [DOI] |
| 23. | Theodosakis N, Micevic G, Sharma R, Baras AS, Lazova R, Bosenberg MW, Rodić N. Integrative discovery of CD98 as a melanoma biomarker. Pigment Cell Melanoma Res. 2016;29:385-387. [PubMed] [DOI] |
| 24. | Quang ND, Ikeda S, Harada K. Nucleotide variation in Quercus crispula Blume. Heredity (Edinb). 2008;101:166-174. [PubMed] [DOI] |
| 25. | Le Vee M, Jouan E, Lecureur V, Fardel O. Aryl hydrocarbon receptor-dependent up-regulation of the heterodimeric amino acid transporter LAT1 (SLC7A5)/CD98hc (SLC3A2) by diesel exhaust particle extract in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2016;290:74-85. [PubMed] [DOI] |
| 26. | Behrens CR, Ha EH, Chinn LL, Bowers S, Probst G, Fitch-Bruhns M, Monteon J, Valdiosera A, Bermudez A, Liao-Chan S. Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs. Mol Pharm. 2015;12:3986-3998. [PubMed] [DOI] |
| 27. | Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382-389. [PubMed] [DOI] |
| 28. | Poettler M, Unseld M, Braemswig K, Haitel A, Zielinski CC, Prager GW. CD98hc (SLC3A2) drives integrin-dependent renal cancer cell behavior. Mol Cancer. 2013;12:169. [PubMed] [DOI] |
| 29. | Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101:173-179. [PubMed] [DOI] |
| 30. | Namikawa M, Kakizaki S, Kaira K, Tojima H, Yamazaki Y, Horiguchi N, Sato K, Oriuchi N, Tominaga H, Sunose Y. Expression of amino acid transporters (LAT1, ASCT2 and xCT) as clinical significance in hepatocellular carcinoma. Hepatol Res. 2014; Oct 9. [Epub ahead of print]. [PubMed] [DOI] |
| 31. | Li J, Qiang J, Chen SF, Wang X, Fu J, Chen Y. The impact of L-type amino acid transporter 1 (LAT1) in human hepatocellular carcinoma. Tumour Biol. 2013;34:2977-2981. [PubMed] [DOI] |
| 32. | Kondoh N, Imazeki N, Arai M, Hada A, Hatsuse K, Matsuo H, Matsubara O, Ohkura S, Yamamoto M. Activation of a system A amino acid transporter, ATA1/SLC38A1, in human hepatocellular carcinoma and preneoplastic liver tissues. Int J Oncol. 2007;31:81-87. [PubMed] [DOI] |