Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.878
Revised: December 18, 2002
Accepted: December 25, 2002
Published online: April 15, 2003
AIM: To summarize the characteristics of patients suffered from primary biliary cirrhosis associated with ulcerative colitis.
METHODS: To report a new case and review the literature.
RESULTS: There were 18 cases (including our case) of primary biliary cirrhosis complicated with ulcerative colitis reported in the literature. Compared with classical primary biliary cirrhosis, the patients were more often males and younger similar. The bowel lesions were usually mild with proctitis predominated. While ulcerative colitis was diagnosed before primary biliary cirrhosis in 13 cases, the presentation of primary biliary cirrhosis was earlier than that of ulcerative colitis in our new case reported here. The prevalence of primary biliary cirrhosis among patients of ulcerative colitis was almost 30 times higher than in general population.
CONCLUSION: Association of primary biliary cirrhosis with ulcerative colitis is rare. It should be considered in the differential diagnosis of hepatobiliary disease in patients with ulcerative colitis, and vice versa.
- Citation: Xiao WB, Liu YL. Primary biliary cirrhosis and ulcerative colitis: A case report and review of literature. World J Gastroenterol 2003; 9(4): 878-880
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/878.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.878
A variety of hepatobiliary diseases have been described in patients with ulcerative colitis (UC). The incidence is 3%-15% with persistent abnormal liver function tests, and up to 90% with abnormal liver histology at surgery or autopsy[1]. These include primary sclerosing cholangitis, pericholangitis of the small bile ducts, chronic active hepatitis, cholangiocarcinoma and cirrhosis. The association of primary biliary cirrhosis (PBC) and UC has occasionally been reported[2-4]. We hereby presented an additional case.
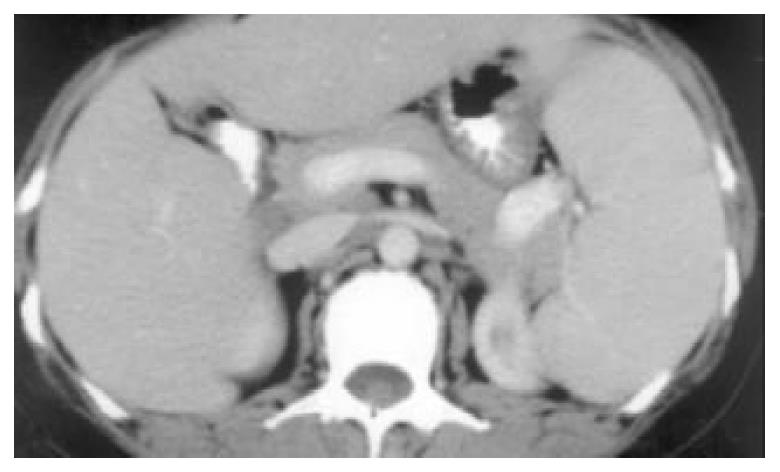
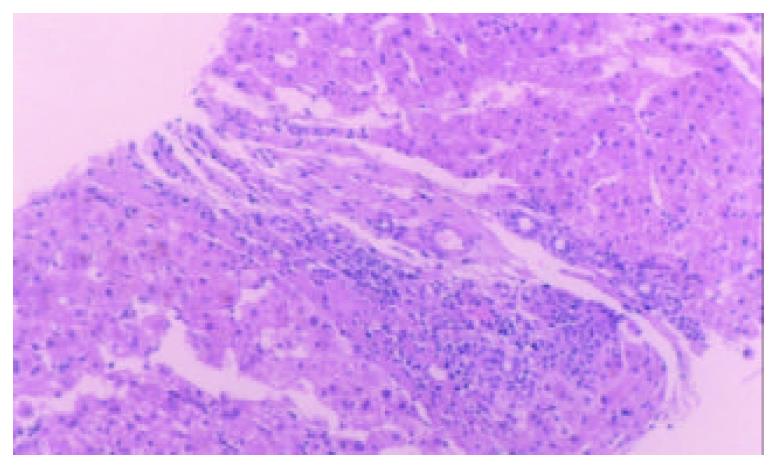
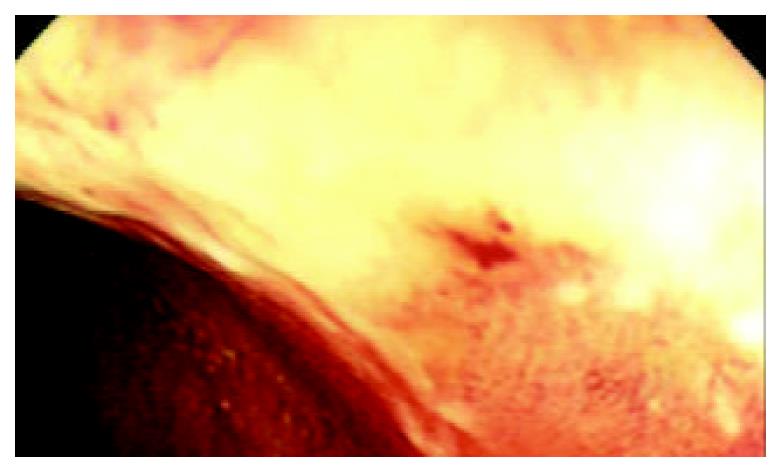
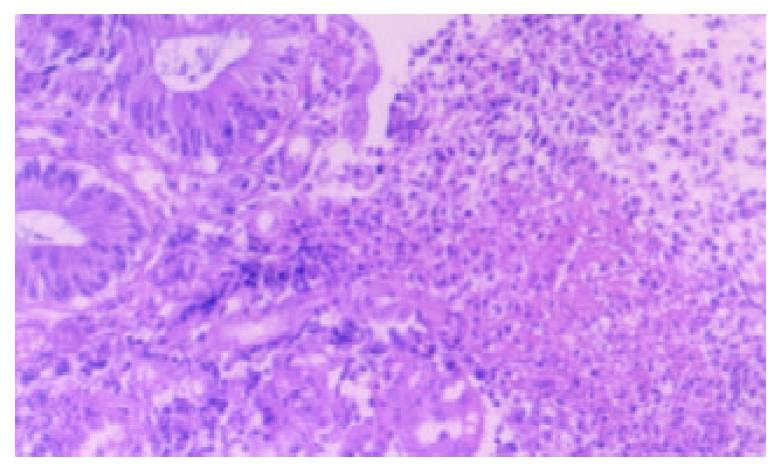
A 50-year-old woman was admitted to our hospital because of skin pruiritus for two years. One year ago she was found to have jaundice and examined in another hospital. The results of all hepatitis virus test were negative, and she was diagnosed as cryptogenic hepatitis. Six months ago she had diarrhea 2-3 times per day. Upon physical examination icterus was evident on the skin and sclera. Hepatomegaly and splenomegaly were found. Blood routine test revealed hemoglobulin 106 g/L, WBC and platelet counts normal. ESR 78 mm/h. IgM elevated to 7.73 g/L, IgA and IgG normal. Liver function tests showed ALT 81 U/L, AST 139 U/L, serum total bilirubin 223.2 umol/L, direct bilirubin 138.3 umol/L and GGT 1042 U/L. Cholesterol was 12.2 mmol/L. Immunological tests, by indirect immunofluorescence were as follows: ANA, 1:10 positive, anti-mitochondria antibody (AMA), negative, anti-LK (anti-liver and anti-kidney) microsome antibody and anti-smooth muscle antibody negative. Hepatomegaly, splenomegaly and portal hypertension were seen in abdominal computed tomography (Figure 1). No intra- and extrahepatic bile duct abnormalities were found by ERCP. Liver biopsy disclosed an increased portal inflitration consisting of lymphocytes, plasmacytes, and scattered eosinophils with fibrosis and pseudolobulation. Two portal areas had hyperplastic ductule. The findings were compatible with PBC stage 3-4 (Figure 2). Colonoscopy showed up to 20 cm continuous lesions of the mucosa with numerous ulcers and adherent mucopurulent exudate (Figure 3). Histological examination demonstrated loss of goblet cells, neutrophilic microabscesses in the crypts, and inflitration with mononuclear cells and plasma cells in the lamina propria (Figure 4). Ulcerative colitis limited to rectosigmoid was diagnosed. Ursodeoxycholic acid (UDCA) was prescribed. One month later serum bilirubin and liver function tests returned to normal. But diarrhea persisted and sulfasalazine was given. The bowel symptoms were then relieved.
Hepatobiliary diseases are commonly found in association with UC. Primary sclerosing cholangitis (PSC) involved the large bile ducts is the most common associated hepatopathology, affecting 2.4%-7.5% of UC patients. PBC is another autoimmune hepatobiliary disease similar to PSC. The typical features include skin pruiritus, with hyperbilirubinemia, increased IgM levels and hypercholesterolemia. AMA is present in about 95% of patients, that is in about 5% AMA is negative. Cholangiogram is normal. Granulomatous inflammation of the periportal areas is a typical finding. The clinical manifestation and histologic features of our patient were compatible with PBC, although AMA was negative.
PBC associated with UC is rare. There were 18 cases (including our case) reported in the literature. In 4 cases from Sweden and Taiwan data were incomplete. In the remaining 14 patients, their presentations were different from that of typical PBC without UC (Table 1). It is well known that PBC usually affects middle-aged women. The sex ratio is 10:1 (women to men), and the mean age at diagnosis was 57.5 years. It is quite obvious that these figures differ in the patients of table 1. The sex ratio was low (2:1) and the mean age was younger similar to that of UC. In addition, UC was mild and limited with proctitis predominating. Only 3 of 12 patients had pancolitis. Similarly, UC associated with PSC tends to be mild, but usually involving the whole bowel.
| Refer-ences | Patient | Sex | Age (yr) | Comorbidity | AMA (titer) |
| 3 | 1 | F | 65 | Pancolitis, chronic | 1/64 |
| pancreatitis | |||||
| 4 | 2 | F | 20 | Proctitis | Strongly |
| 4 | 3 | M | 22 | Proctitis | 1/160 |
| 4 | 4 | M | 44 | Proctitis | 1/320 |
| 4 | 5 | F | 28 | Left-side colitis | 1/200 |
| 5 | 6 | F | 49 | Proctitis, chronic | Positive |
| myelocytic leukemia | |||||
| 6 | 7 | F | 45 | Proctitis,chronic | Positive |
| pancreatitis | |||||
| 7 | 8 | M | 40 | Left-side colitis | 1/160 |
| 8 | 9 | F | 48 | Pancolitis, renal | 1/160 |
| cell carcinoma | |||||
| 9 | 10 | M | 58 | Proctitis | 1/160 |
| 9 | 11 | F | 68 | Proctitis | 1/160 |
| 10 | 12 | F | 43 | Pancolitis | > 1/160 |
| 11 | 13 | M | 61 | Proctitis | Positive |
| Present | 14 | F | 50 | Proctitis | Negative |
The prevalence of PBC in Europe is about 15/100000. In a cohort of 412 cases of UC, 2 cases of PBC were diagnosed estimating the prevalence of PBC among these patients 485/1001000, almost 30 times higher as compared with the general population[2]. It is believed that both PBC and UC are autoimmune disease, and have similar pathogenesis. Associated autoimmune conditions are frequent in both PBC and UC. PBC and UC may be an additional example of a true association between syndromes of autoimmune etiology. But the detail remains to be clarified. In 13 out of the 14 patients UC were diagnosed many years before the diagnosis of PBC. Symptoms of PBC usually presented during the active stage of UC. In other words, PBC runs a course depending the activity of UC. So most authors considered PBC as the extraintestinal (hepatobiliary) manifestiation of UC. But in our case it is clear that the presentation of PBC is earlier than that of UC. We believe that UC also could be regarded as extrahepatic manifestation of PBC.
Treatment of these patients presents some difficulties. Prednisolone, which is indicated for the treatment of the active stage of UC, might lead to extensive osteroporosis in PBC. But as mentioned above, UC associated with PBC is usually mild, so sulfasalazine is the first choice. If the presentation of PBC is evident, UDCA could be added.
In conclusion the reported association between UC and PBC is rare, and it has not yet been established whether it occurs coincidently or is due to a common immunogenetic basis. PBC should be considered in the differential diagnosis of hepatobiliary disease in patients with UC, and vice versa. Liver biopsy and serum AMA are two diagnostic criteria that clearly distinguish PBC from PSC.
Edited by Xu JY
| 1. | Monsén U, Sorstad J, Hellers G, Johansson C. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711-716. [PubMed] [Cited in This Article: ] |
| 2. | Chien RN, Sheen IS, Chen TJ, Liaw YF. [A clinicopathological study in primary biliary cirrhosis]. J Formos Med Assoc. 1992;91 Suppl 2:S117-S121. [PubMed] [Cited in This Article: ] |
| 3. | Kato Y, Morimoto H, Unoura M, Kobayashi K, Hattori N, Nakanuma Y. Primary biliary cirrhosis and chronic pancreatitis in a patient with ulcerative colitis. J Clin Gastroenterol. 1985;7:425-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Bush A, Mitchison H, Walt R, Baron JH, Boylston AW, Summerfield JA. Primary biliary cirrhosis and ulcerative colitis. Gastroenterology. 1987;92:2009-2013. [PubMed] [Cited in This Article: ] |
| 5. | Akahoshi K, Miyata Y, Hashimoto M, Koga S, Chijiiwa Y, Misawa T, Suematsu E, Nishimura J, Nawata H. A case of combined primary biliary cirrhosis, ulcerative colitis and chronic myelocytic leukemia. Gastroenterol Jpn. 1992;27:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Veloso FT, Dias LM, Carvalho J, Fraga J, Saleiro J. Ulcerative colitis, primary biliary cirrhosis, and chronic pancreatitis: coincident or coexistent. J Clin Gastroenterol. 1993;16:55-57. [PubMed] [Cited in This Article: ] |
| 7. | Lever E, Balasubramanian K, Condon S, Wat BY. Primary biliary cirrhosis associated with ulcerative colitis. Am J Gastroenterol. 1993;88:945-947. [PubMed] [Cited in This Article: ] |
| 8. | Satsangi J, Marshall J, Roskell D, Jewell D. Ulcerative colitis complicated by renal cell carcinoma: a series of three patients. Gut. 1996;38:148-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Koulentaki M, Koutroubakis IE, Petinaki E, Tzardi M, Oekonomaki H, Mouzas I, Kouroumalis EA. Ulcerative colitis associated with primary biliary cirrhosis. Dig Dis Sci. 1999;44:1953-1956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ohge H, Takesue Y, Yokoyama T, Hiyama E, Murakami Y, Imamura Y, Shimamoto F, Matsuura Y. Progression of primary biliary cirrhosis after proctocolectomy for ulcerative colitis. J Gastroenterol. 2000;35:870-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Nakayama M, Tsuji H, Shimono J, Azuma K, Ogata H, Matsumoto T, Aoyagi K, Fujishima M, Iida M. Primary biliary cirrhosis associated with ulcerative colitis. Fukuoka Igaku Zasshi. 2001;92:354-359. [PubMed] [Cited in This Article: ] |