INTRODUCTION
Dendritic cells (DCs) play critical roles in the initiation and modulation of immune responses and may determine the balance between tolerance and immunity[1-3]. Evidence has demonstrated that immunosuppressive cytokines modified DCs favour the induction of alloantigen-specific T cells anergy and prolong allograft survival. To date, there have been only a few reports of genetic manipulation of DCs for therapeutic application in organ transplantation. Retroviral and adenoviral gene transfer methods, for example, have been used to express immunosuppressive molecules such as vIL-10, TGF-β by DC[4,5]. In the present study, we demonstrated the feasibility of liposome transduction of DC to express hIL-10. In addition, we tested the in vivo ability of IL-10-DC to prevent small bowel graft rejection in rat intestinal transplantation model.
MATERIALS AND METHODS
Animals
Healthy adult Sprague-Dawley (SD) and Wistar-Furth rats, weighing 200-250 g, were used as donors and recipients, respectively. They were obtained from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
DC propagation and transfection
Splenocyte suspensions from SD rats were prepared in RPMI1640 complete medium (Gibco/BRL), supplemented with 10% v/v heat-inactivated fetal calf serum (Hyclone) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/mL, R&D) and IL-4 (10 ng/mL, R&D). Cultures were fed every 3 d by complete medium exchange including cytokines. After 6-8 d of culturing, when the adherent cells presented with primitive dendrites observed under electronic microscope, DC was propagated successfully. pcDNA3/hIL-10 vector encoding human IL-10 cDNA sequence was transfected to DC by the liposome method using the Lipofectamin 2000 kit (Gibco/BRL) according to the manufacturer’s instructions and the vector without insert was also transduced as a control. After 10-14 d in selective medium, DCs were then selected for geneticin-resistant colonies.
ELISA
The supernatant from transfected DCs was assayed for IL-10 expression by ELISA kit (Genzyme). The clone that gave the maximum ELISA reading was chosen for use in in vivo and in vitro experiments.
Mixed leukocyte reaction (MLR)
The ability of transduced DC to stimulate naive allogeneic T cells, assayed by using MTT (Sigma) method, was determined by MLR. Various numbers (2 × 103-2 × 105/well) of DC and IL-10-DC, pretreated with mitomycin C (25 μg/mL, Amresco), were cocultured with allogenic T-cells from Wistar rats as responders (2 × 105/well) at a ratio of 1:1, 1:10 and 1:50 for 72 h in 0.2 mL RPMI-1640 complete medium in a 96-well U-bottomed microtiter plate. The results monitored by a microtiter plate reader (Bio-Rad, Tokyo, Japan) at 570 nm, were expressed as inhibition rate and calculated according to the following equation: inhibition rate = 1 - (mean OD570 value of experiment group/mean OD570 value of control group) × 100%.
Flow cytometric analysis
At the indicated time point, supernatant cells were washed twice in cold PBS and then resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. One hundred μL of the solution (1 × 105 cells) was transferred to a 5 mL culture tube, then 5 μL of FITC-Annexin V and 10 μL of propidium iodide (Gibco/BRL) were added. The cells were gently vortexed and incubated for 15 min at room temperature in the dark and subjected to FACS. Those cells with negative propidium iodide staining and positive annexin V staining were considered as the proportion of cells actively undergoing apoptosis.
Experimental group and intestinal transplantation
All rats were randomized into three groups and each group had six pairs of rats. These groups were treated with a single intravenous injection of IL-10-DCs (2 × 106), untransduced DCs (2 × 106) or empty plasmid through tail vein seven days before intestinal transplantation. Operation procedures were performed under ether anesthesia. The small intestine was transplanted according to the modified procedure described by Monchik et al[6]. In brief, after an overnight fast, a 10-cm segment of proximal jejunum or distal ileum, including the superior mysenteric artery and vein, was removed from the donor after ligation of small mysenteric vessel branches and intravascular irrigation with cold heparinized saline solution. The graft was kept in 4 °C cold Ringer’s solution until transplantation. The recipient abdomen was opened and the graft was reperfused by end-to-side anastomosis of the superior mesenteric artery and vein to the recipient’s abdominal aorta and inferior vena cava under operative microscope, respectively. Both ends of the graft were then exteriorized through the right abdominal wall as stomas, isolating the segment from the recipient’s native gastrointestinal tract. Death due to technical failure that occurred within 72 h of operation was excluded from the study.
Histological examination
The rats were sacrificed and used for histological examination when recipients showed clinical signs of rejection[7,8]. After fixed in 10% buffered formalin, embedded in paraffin, samples were stained with hematoxylin and eosin and examined by light microscope. Three samples were obtained at the time of donor operation and used as normal group for histological study.
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analyses were performed using Student’s t test. Probability values of less than 0.05 were considered significant.
RESULTS
IL-10-DCs induced allospecific T cell hyporesponsiveness
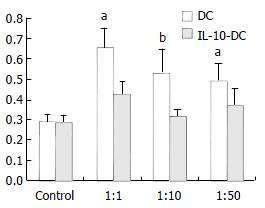
We examined the responses of naïve allospecific T cells to IL-10-DCs. As shown in Figure 1, IL-10-DCs could suppress allogeneic MLR compared with that of untransduced DCs. The latter were potent stimulators of allogeneic T-cell proliferation. The inhibitory effect was most striking with the stimulator/effector (S/E) ratio of 1:10. The inhibition rates were 33.25%, 41.19% and 22.92% with the S/E ratio of 1:1, 1:10 and 1:50 respectively (Figure 1).
Figure 1 Inhibitory effect of IL-10-DC on allogenic T cell proliferation.
aP < 0.05, bP < 0.01, vs DC group.
Apoptosis assay by flow cytometric analysis
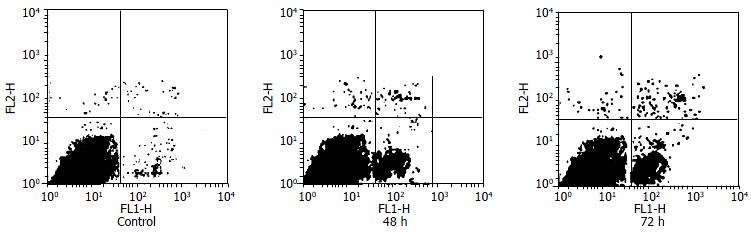
Supernatant T cells cocultured with IL-10-DCs in MLR underwent apoptosis. At 48 h and 72 h by flow cytometry counting apoptotic T cells were 13.8% and 30.1% with the S/E ratio of 1:10, but control group did not undergo significant apoptosis (P < 0.05) (Figure 2).
Figure 2 Flow cytometric analysis for apoptotic T cells induced by IL-10-DC.
Allograft survival and histological evaluation
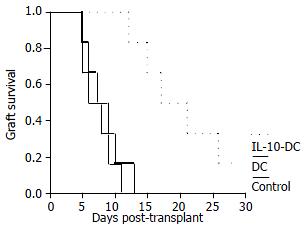
Untransduced DCs pretreatment did not affect allograft survival and the recipients pretreated with IL-10-DCs had a moderate allograft prolongation. Mean allograft survival in IL-10-DCs group was 19.8 ± 6.3 d (P < 0.01), compared with 7.3 ± 2.4 d in control group and 8.3 ± 2.9 d in untransduced DCs group (Figure 3). Although untreated recipient on postoperative day (POD) 3 showed acute rejection with blunted villi, the number of goblet cells was decreased and a large amount of inflammatory cell infiltration occurred in lamina propria mucosa, the grafts treated with IL-10-DCs demonstrated normal intestinal mucosa negative for rejection on POD 5 and mild lymphocyte infiltration and blunting of villi with edema on POD 7 (Figure 4).
Figure 3 Survival (%) of recipient small intestines from control group (n = 6), untransduced DC group (n = 6) and IL-10-DC group (n = 6).
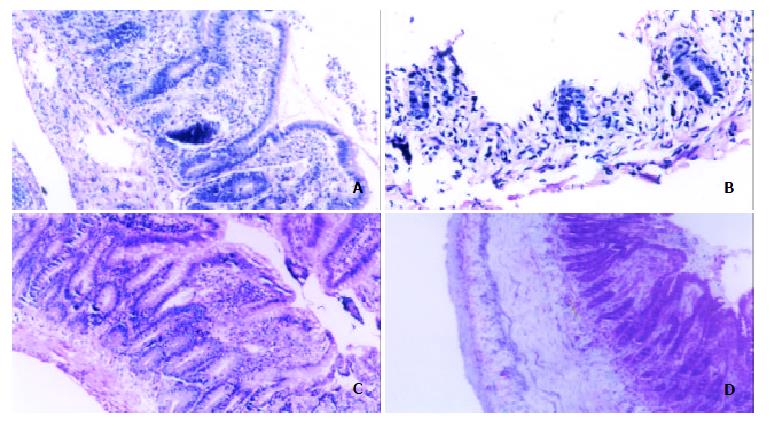
Figure 4 Histological comparison of allografts between control group rats and IL-10-DC pretreated rats.
Control group showed acute rejection sign on POD 3 (A) and mucosal sloughing and necrosis and destruction of normal glandular architecture on POD 7 (B). IL-10-DC group demonstrated mild lymphocyte infiltration and blunting of villi with edema on POD 7 (C) and fibroblastic proliferation extending from the submucosa to the lamina muscularis (D). (hematoxylin-erosin staining, × 200).
DISCUSSION
Despite improvements in the posttransplantation immunosuppressive therapeutic regimens, the management of allograft rejection has been still dependent on the use of nonspecific immunosuppressive agents[9], which could prevent the host from complications, such as infections and lymphoproliferative disorders[10,11]. In this regard, the successful induction of donor-specific immune tolerance remains a major challenge in organ transplantation. However, the lymphoid-rich small intestinal allograft might differ from such lymphoid-poor organs as the heart and kidney, making tolerance induction a particularly troublesome approach specific to this organ[12-14]. An approach to modify donor-derived DC in order to induce an immunological hyporesponsive state in the recipient has been attractive as a potential strategy in transplantation as it would theoretically let the possibility of donor-specific tolerance come true[15,16].
IL-10 has been regarded as an immunosuppressive cytokine because of its ability to down-regulate the synthesis of a broad spectrum of proinflammatory cytokines by DCs, monocytes or macrophages, and to inhibit allogeneic proliferative responses in vitro[17-19]. It is for these reasons that IL-10 has been considered as a potential means in the induction of tolerance. In the immature state, DC could express low levels of CD80, CD86 and co-stimulatory molecules that are essential for the amplification of immune response to foreign peptides[16,20-22]. IL-10 has been shown to block DC maturation in vitro[23,24]. Our approach was therefore to investigate strategies that might delay maturation of DC to prolong their immature phenotype. In our studies we found that these IL-10-DCs were less stimulatory in the MLR compared with untransduced DCs. One of the major mechanisms by which IL-10 inhibited DC antigen presenting function was to down-regulate MHC class II and costimulatory molecule expression on DC[25].
Our data confirmed that IL-10-DC could inhibit the proliferation of allogeneic T cells and induce these cell apoptosis, which may be related to the prolonged survival of graft. It has been accepted that the initiation of T-cell responses to grafted tissues requires two distinct signals[26-28]. The essential signal is the engagement of T cell receptors (TCR) to antigen peptide in the content of major histocompatibility complex molecules on antigen presenting cells. Other costimulatory receptor-ligand interactions between T cell and APC are needed. Signals through the TCR alone could lead to allospecific T-cell anergy or apoptosis[29]. Schartz[30] has demonstrated that lack of second signal would result in increased amounts of a negative regulatory factor, Nil-2a, in anergic human T cells. This factor has been shown to suppress IL-2 nuclear transcription factors of AP-1 and NF-κB. NF-κB could inhibit apoptosis by induction or up-regulation expression of anti-apoptotic gene such as Bcl-2. Therefore, the suppression of NF-κB activation could give rise to decreased IL-2 expression and T cell apoptosis[31,32].
Our studies were consistent with previous studies, perhaps not surprisingly, showing that IL-10-DC could exert tolerogenic effects on organ transplantation[25,33]. In IL-10-DC group, median intestine allograft survival time was 19.86.3 d. Compared with control group (7.3 ± 2.4 d) and untransduced DC group (8.3 ± 2.9 d), the survival time was 2.5-fold longer. There are two possible explanations for the lack of long- term survival in our study. One is that IL-10-DC might not survive long enough to induce a permanent state of tolerance. Long-term survival of IL-10-DC in vivo would be particularly important if apoptosis induction of allogeneic T cells was a major mechanism of allograft survival. The other, as far as long-term tolerance is concerned, is that the level of expression of IL-10 by IL-10-DC was still not high enough to affect the recipient immune modulation for all alloreactive T cells, though the level of IL-10 expression was higher than that in normal condition. For intestine, considering its high immunogenicity, it would be necessary to enhance gene transfer efficiency and transgene expression level[34].
In conclusion, these studies demonstrate the feasibility of immunosuppressive gene delivery system. We believe that this highly targeted method of inducing tolerogenicity of donor derived DC may reduce the need for recipient nonspecific immunosuppression and play an important role in clinical strategies of tolerance induction.