Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.1108
Revised: April 12, 2002
Accepted: April 20, 2002
Published online: December 15, 2002
AIM: To investigate the pathogenic mechanism of Hirschsprung’s disease (HD) at the molecular level and to elucidate the relationship between RET oncogene and Chinese patients with HD.
METHODS: Exon 13 of RET oncogene from 20 unrelated HD patients was analyzed with polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP). The positive amplifying products were then sequenced. According to the results of SSCP and DNA sequence, SSCP was done as well for the samples from the family other members of some cases with mutated RET gene.
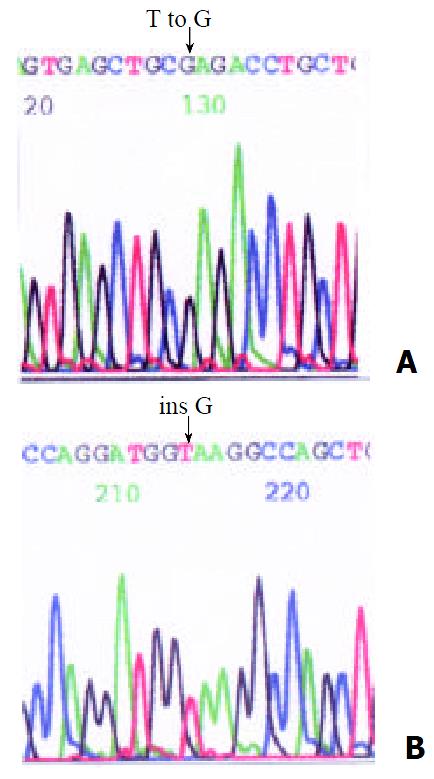
RESULTS: SSCP analysis indicated that mobility abnormality existed in 4 unrelated HD patients. Direct DNA sequence analysis identified a missense mutation, T to G at the nucleotide 18888 and a frameshift mutation at the nucleotide 18926 insG. In a HD family, the sicked child and his father were the same heterozygous missense mutation (T to G at nucleotide 18888).
CONCLUSION: Among Chinese HD patients, RET gene mutations may exist in considerable proportion with different patterns. These new discoveries indicate that RET mutations may play an important role in the pathogenesis of unrelated HD in the Chinese population. PCR-SSCP combined with DNA sequence can be used as a tool in the genetic diagnosis of HD.
- Citation: Li JC, Ding SP, Song Y, Li MJ. Mutation of RET gene in Chinese patients with Hirschsprung’s disease. World J Gastroenterol 2002; 8(6): 1108-1111
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/1108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.1108
Hirschsprung’s disease (HD), with the incidence of 1/5000, occupies the second in the congenital malformation, manifests as complete or incomplete ileus clinically[1-5]. As a complex disease, HD has been ascribed to the absence in the terminal hindgut of ganglion cells from the neural crest, which causes the abnormal contraction of involved intestines, and then the proximal end of the sick colon appears compensatory dilated thickness and forms megacolon. However, the reason for the deficit of ganglion cells remains in dispute[5]. With the development of the molecular biology, the molecular pathogenesis of HD have attracted the attention of many scholars. In 1993, Genetic mapping in multiplex families and the mutational analysis of candidate genes have led to the definitive identification of the defects that contribute to the HD risk. It has been found that the gene defects present in a major proportion of Hirschsprung’s disease families are mutations in chromosome 10q11.2, which have now been found to be associated with RET gene[6-9]. Subsequently, various kinds of mutation of RET gene have been reported abroad[10-19]. However, there existed less reports about HD in Chinese population. In order to further investigate the pathogenic mechanism of HD, we examined exon 13 mutations of RET gene in 20 unrelated patients with the single strand conformation ploymorphism analysis of polymerase chain reaction products (PCR-SSCP).
Twenty unrelated cases with HD by pathological verification were collected after operation at Zhejiang Children’s Hospital during 1998 to 2000. Four milliliters of peripheral blood samples used for the experiment were obtained from each patient and the control blood samples were taken from anonymous donors provided by Zhejiang Children’s Hospital. The blood samples were anti-coagulated by sodium citrate and DNA was extracted according to the standard protocols.
The designed primers were synthesized by Shanghai Shenggong Biology Company. The primer sequence of exon 13: (Forward) 5’-GACCTGGTATGGTCATGGA-3’, (Reverse) 5’-AAGAGGGAGAACAGGGCTGTA-3’. The PCR mixture contained 200 ng of template-DNA and PCR reaction buffer containing 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.4), 1.5 mmol/L MgCl2, 0.5 μmol/L each of two fragment-specific primers, 100 μmol/L each of dATP, dGTP, dTTP and dCTP, and 2 units of Taq DNA polymerase (provided by Shanghai Shenggong Biology Company) for a reaction volume of 50 μL. The conditions for temperature cycling for all PCR amplifications were 94 °C for 5 min for pre-denaturation, 94 °C for 45 second, 58 °C for 45 second and 72 °C for 45 second. Amplifications were carried out for 30 cycles with a final extension for 10 min at 72 °C. The amplified fragments were run in 1% agarose gel, and were confirmed to be 253bp in size using 100-bp ladder markers.
SSCP analysis of fragments was performed on a Mini Electrophoresis Unit (Bio-Rad Company, U.S.A). 10 uL of the PCR product was diluted with 10 uL of sample buffer containing 90% formamide, 0.05% Bromphenol Blue dye and 0.05% xylene cyanol. The samples were heated at 100 °C for 8 min, transferred into an ice-cold water bath for 3 min, and analysed by 8% PAGE in 45 mM-Tris-borate (pH8.0)/1 mM-EDTA (TBE) buffer under 13 v·cm-1 at 10 °C.
Gels were stained with silver as follows: fixed in 100 mL·L-1 alcohol for 10 min→oxidized in 100 mL·L-1 nitric acid for 3 min→drip washed for 1 min with double distilled water→stained in 2 g·L-1 silver nitric acid for 5 min→drip washed for 1 min with double distilled water→showed appropriated color in 15 g·L-1 anhydrous sodium carbonate and 4 mL·L-1 formalin→ended reducing response by 7.5 mL·L glacial acetic acid→ drip washed with double distilled water→analysis results and photographed.
Abnormal PCR products screened by SSCP was cut from gel and purified by VIOGENE kit. Agarose gel-purified PCR products was subcloned to pUCm-T vector through TA clone. Sequence analysis was carried out with a PE377 automated sequencer.
The increment of all DNA samples from HD patients was a single strand with the length of 253 bp, and so was that from normal control, which indicated that a large fragment insertion and deletion did not exist in the region of exon 13 of RET gene among 20 HD patients.
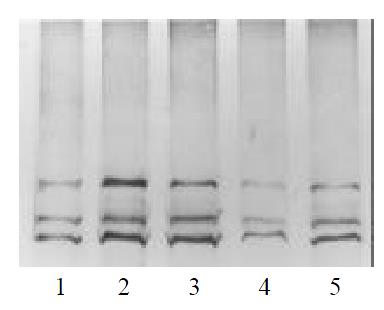
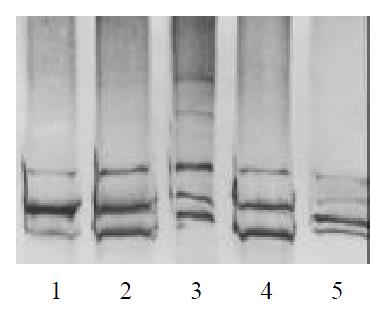
Of all the patients and normal control analyzed, four showed abnormal SSCP patterns in exon 13. In Figures 1 and 2, the mobility of one single-strand was abnormal in case 2,3,4. In case 6, the mobility of two single-strand was abnormal. DNA sequence analysis showed nucleotide changes in all variable SSCP bands. Of the two patterns of nucleotide change, one was missense mutation, and the other was frameshift mutation (Figure 3, Table 1).
| case | he heterozygous of allele gene | Nucleotide change | Amino acid change | Mutation types |
| case2 | Heterozygote | 18888 T→G | Cys→Phe | missense mutation |
| case3 | Heterozygote | 18888 T→G | Cys→Phe | missense mutation |
| case4 | Heterozygote | 18888 T→G | Cys→Phe | missense mutation |
| case6 | Homozygote | 18926 ins G | -- | frameshift mutation |
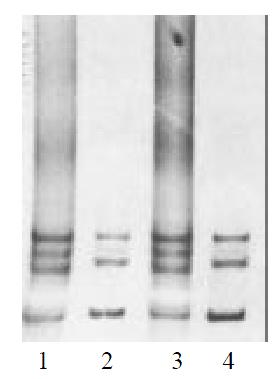
The parents genotypes of the case 2, 3 and 4 were also examined by SSCP. We found that the mobility pattern of the father was in coincidence with his son in case 4, while the mobility pattern of the mother was normal (Figure 4). DNA sequence analysis showed the same mutation between the father and the son, implied that the exon 13 of RET gene in case 4 was originated from his paternal heredity. No abnormal patterns of DNA migration was observed in the parents of the case2, 3.
The human RET gene lies on chromosome band 10q11.2 and comprises 20 exons, with the length about 80 kb[20-26]. The RET gene encodes a receptor tyrosine kinase consisting of an intracellular tyrosine kinase domain, a transmembrane domain and an extracellular domain which includes a “cadherin-like” region. Receptor tyrosine kinases generally function as ligand dependent dimers, which phosphorylate “second messenger” proteins in the cytoplasm and they are commonly associated with regulation of cell growth and differentiation, the development of normal nerves, and expressed in gangliogenic source cells (such as neurogenic ganglia and ganglia of peripheral nerve system, neuroendocrine cells, epidermic pigment cells, etc.). Mutations of the RET gene may lead to the premature termination of the transcription and translation procedures or the alteration of amino acid sequence, and thus, during the embryogenesis, the signal conduction was obstructed, causing the nerve cells migration stagnated and the colonic nerves defected.
Exon 13 of RET gene played a key role in encoding the tyrosine kinase domain and therefore the exon 13 on 20 unrelated Chinese HD patients was examined with PCR-SSCP in the present study. The PCR result revealed that the increment of all DNA samples from HD patients was a single strand with the same length of 253 bp as that of the samples from the control, which indicated that a large fragment insertion and deletion did not exist in the exon 13 region among 20 HD cases. The SSCP analysis indicated that the mobility abnormality existed in 4 cases and further DNA sequencing analysis exhibited two novel mutations: a transition, T to G at the nucleotide 18888, which lead to a Cysteine transformed phenylalanine and the disulfide bond was disrupted of the receptor tyrosine kinase. And thus the RET protein was destroyed and the signal conduction was obstructed which finally caused HD. Another frameshift insert G at the nucleotide 18926, which could altered the amino acid sequence and thus Hirschsprung’s disease arose from the abnormal RET protein as mentioned above. In 4 HD cases, among which 3 cases were heterozygous mutation and 1 case who was two-day-old child was homozygous mutation, accompanied by serious icterus. It has been reported that only a half quantity of RET gene mutation are likely to cause HD and homozygous mutation of RET gene may be fetal in human[24-26], which has been confirmed by our finding.
So far, a variety of frameshift, nonsense, or missense mutations scattered along the entire RET proto-oncogene have been identified in HD patients. However, the ‘hot spot’ region has not been found, which causes the difficulties in the design of specific primer and the detection of the RET gene mutation by routine diagnostic method. Single-strand conformation polymorphism (SSCP) analysis enables the discrimination of DNA fragments of the same size containing sequence variations and has been employed in the investigation of the monogenic and polygenic diseases as well as the oncogene and anti-oncogene studies[27-38]. The technique is based on the facts that partially denatured double stranded DNA (dsDNA) migrates as two single stranded DNA (ssDNA) in non-denaturing polyacrylamide gel edectrophoresis (PAGE), and that small changes in the nucleotide sequence may alter the ssDNA conformation and therefore its electrophoretic profiles. Thus, the single-strand conformational polymorphism analysis is useful to screen out the mutations in a small region with 150-500 bp in size on a gene of interest with the rapid, economic and sensitive characteristics. Because of the fragment of RET gene exon 13 being 253 bp, it is therefore suitable to take the PCR-SSCP analysis in our experiment. Furthermore, we found that increasing the concentrations of acrylamide and methylene bisacrylamide, using the thin gel (0.75 mm-thick) and applying the 100 mL·L-1 glycerin made the straps obtained more be clear and distinguishable.
Hirschsprung’s disease is considered a heterogeneous genetic disorder with dominant, recessive, sex-linked and polygenic forms, and associated with a number of other genetic disorders including Down’s syndrome[39], familial leukoplakia, congenital central dysfunction of ventilation, etc. Up to now, it has not been reported yet on the inheritance patterns in the Chinese patients with HD. In the present study, SSCP analysis was performed as well for the samples from the family members of some cases with mutated RET gene. The results showed that in a HD family, the sicked child and his father were the same heterozygous missense mutation (Cys→Phe). However, the inheritance patterns for the family remain to be investigated. Besides, the sequencing analysis proved that even the same exon 13 on the RET gene manifested the allele heterogeneity in the sites and ways of mutation (missense mutation and frameshift mutation). Therefore, congenital megacolon is a highly heterogenous disease.
At present, the clinical therapy for Hirschsprung’s disease includes early diagnosis and excision of involved intestine segments[40-42]. But as for the patients with the long segment or the whole colon involved, the effect of the surgical intervention is extremely poor. In the present case, there were 4 cases of mutation screened out by the exon 13 on RET gene from 20 HD cases. The mutation rate of RET gene was 20%. Considering the false-negative result by SSCP analysis, the rate of the RET mutation should be higher. The higher mutation rate of RET gene implied that the checkout of gene mutation can be taken as a routine technique of molecular genetics for diagnosing congenital megacolon; together with the chromosome aberration analysis, the method can be applied in the clinical prenatal diagnosis.
Edited by Zhu L
| 1. | Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Li JC, Mi KH, Zhou JL, Busch L, Kuhnel W. The development of colon innervation in trisomy 16 mice and Hirschsprung's disease. World J Gastroenterol. 2001;7:16-21. [PubMed] [Cited in This Article: ] |
| 3. | Li J, Busch LC, Kunel W. [The development of the enteric nervous system in terisomy 16 mice with the occurrence of congenital megacolon]. Zhonghua Yixue Zazhi. 1999;79:466-469. [PubMed] [Cited in This Article: ] |
| 4. | Won KJ, Torihashi S, Mitsui-Saito M, Hori M, Sato K, Suzuki T, Ozaki H, Karaki H. Increased smooth muscle contractility of intestine in the genetic null of the endothelin ETB receptor: a rat model for long segment Hirschsprung's disease. Gut. 2002;50:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Martucciello G, Ceccherini I, Lerone M, Jasonni V. Pathogenesis of Hirschsprung's disease. J Pediatr Surg. 2000;35:1017-1025. [PubMed] [Cited in This Article: ] |
| 6. | Munnes M, Fanaei S, Schmitz B, Muiznieks I, Holschneider AM, Doerfler W. Familial form of hirschsprung disease: nucleotide sequence studies reveal point mutations in the RET proto-oncogene in two of six families but not in other candidate genes. Am J Med Genet. 2000;94:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 7. | Davenport MP, Ward RL, Hawkins NJ. The null oncogene hypothesis and protection from cancer. J Med Genet. 2002;39:12-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kerstjens-Frederikse WS, Hofstra RM, van Essen AJ, Meijers JH, Buys CH. A Hirschsprung disease locus at 22q11. J Med Genet. 1999;36:221-224. [PubMed] [Cited in This Article: ] |
| 9. | Borrego S, Ruiz A, Saez ME, Gimm O, Gao X, López-Alonso M, Hernández A, Wright FA, Antiñolo G, Eng C. RET genotypes comprising specific haplotypes of polymorphic variants predispose to isolated Hirschsprung disease. J Med Genet. 2000;37:572-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Gath R, Goessling A, Keller KM, Koletzko S, Coerdt W, Müntefering H, Wirth S, Hofstra RM, Mulligan L, Eng C. Analysis of the RET, GDNF, EDN3, and EDNRB genes in patients with intestinal neuronal dysplasia and Hirschsprung disease. Gut. 2001;48:671-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sakai T, Nirasawa Y, Itoh Y, Wakizaka A. Japanese patients with sporadic Hirschsprung: mutation analysis of the receptor tyrosine kinase proto-oncogene, endothelin-B receptor, endothelin-3, glial cell line-derived neurotrophic factor and neurturin genes: a comparison with similar studies. Eur J Pediatr. 2000;159:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Julies MG, Moore SW, Kotze MJ, du Plessis L. Novel RET mutations in Hirschsprung's disease patients from the diverse South African population. Eur J Hum Genet. 2001;9:419-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Inoue K, Shimotake T, Tomiyama H, Iwai N. Mutational analysis of the RET and GDNF gene in children with hypoganglionosis. Eur J Pediatr Surg. 2001;11:120-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Shimotake T, Go S, Inoue K, Tomiyama H, Iwai N. A homozygous missense mutation in the tyrosine E kinase domain of the RET proto-oncogene in an infant with total intestinal aganglionosis. Am J Gastroenterol. 2001;96:1286-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Sancandi M, Ceccherini I, Costa M, Fava M, Chen B, Wu Y, Hofstra R, Laurie T, Griffths M, Burge D. Incidence of RET mutations in patients with Hirschsprung's disease. J Pediatr Surg. 2000;35:139-142; discussion 142-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Myers SM, Salomon R, Goessling A, Pelet A, Eng C, von Deimling A, Lyonnet S, Mulligan LM. Investigation of germline GFR alpha-1 mutations in Hirschsprung disease. J Med Genet. 1999;36:217-220. [PubMed] [Cited in This Article: ] |
| 17. | Inoue K, Shimotake T, Inoue K, Tokiwa K, Iwai N. Mutational analysis of the RET proto-oncogene in a kindred with multiple endocrine neoplasia type 2A and Hirschsprung's disease. J Pediatr Surg. 1999;34:1552-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Borrego S, Sáez ME, Ruiz A, Gimm O, López-Alonso M, Antiñolo G, Eng C. Specific polymorphisms in the RET proto-oncogene are over-represented in patients with Hirschsprung disease and may represent loci modifying phenotypic expression. J Med Genet. 1999;36:771-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Pigny P, Bauters C, Wemeau JL, Houcke ML, Crepin M, Caron P, Giraud S, Calender A, Buisine MP, Kerckaert JP. A novel 9-base pair duplication in RET exon 8 in familial medullary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:1700-1704. [PubMed] [Cited in This Article: ] |
| 20. | Lui VC, Samy ET, Sham MH, Mulligan LM, Tam PK. Glial cell line-derived neurotrophic factor family receptors are abnormally expressed in aganglionic bowel of a subpopulation of patients with Hirschsprung's disease. Lab Invest. 2002;82:703-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Zhan J, Xiu Y, Gu J, Fang Z, Hu XL. Expression of RET proto-oncogene and GDNF deficit in Hirschsprung's disease. J Pediatr Surg. 1999;34:1606-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Iwashita T, Kurokawa K, Qiao S, Murakami H, Asai N, Kawai K, Hashimoto M, Watanabe T, Ichihara M, Takahashi M. Functional analysis of RET with Hirschsprung mutations affecting its kinase domain. Gastroenterology. 2001;121:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Lesueur F, Corbex M, McKay JD, Lima J, Soares P, Griseri P, Burgess J, Ceccherini I, Landolfi S, Papotti M. Specific haplotypes of the RET proto-oncogene are over-represented in patients with sporadic papillary thyroid carcinoma. J Med Genet. 2002;39:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Melillo RM, Santoro M, Ong SH, Billaud M, Fusco A, Hadari YR, Schlessinger J, Lax I. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol Cell Biol. 2001;21:4177-4187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Hansford JR, Mulligan LM. Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet. 2000;37:817-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Mograbi B, Bocciardi R, Bourget I, Juhel T, Farahi-Far D, Romeo G, Ceccherini I, Rossi B. The sensitivity of activated Cys Ret mutants to glial cell line-derived neurotrophic factor is mandatory to rescue neuroectodermic cells from apoptosis. Mol Cell Biol. 2001;21:6719-6730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zhu Y, Wang D, Sugimura H. [Mutation analysis of tumor suppressor gene PTEN in bone and soft tissue tumors]. Zhonghua Yixue Zazhi. 2001;81:715-718. [PubMed] [Cited in This Article: ] |
| 28. | Xiao J, Tang B, Xia J. [PCR in the gene diagnosis of Charcot-Marie-Tooth disease]. Zhonghua Yixue Zazhi. 2001;81:138-141. [PubMed] [Cited in This Article: ] |
| 29. | Qin Y, Li B, Tan YS, Sun ZL, Zuo FQ, Sun ZF. Polymorphism of p16INK4a gene and rare mutation of p15INK4b gene exon2 in primary hepatocarcinoma. World J Gastroenterol. 2000;6:411-414. [PubMed] [Cited in This Article: ] |
| 30. | Gong K, Zhang Z, Xin D. [Frequent somatic mutations of the von Hippel-Lindau tumor suppressor gene in primary sporadic human renal clear cell carcinomas]. Zhonghua Yixue Zazhi. 2001;81:142-144. [PubMed] [Cited in This Article: ] |
| 31. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [PubMed] [Cited in This Article: ] |
| 32. | She FF, Su DH, Lin JY, Zhou LY. Virulence and potential pathogenicity of coccoid Helicobacter pylori induced by antibiotics. World J Gastroenterol. 2001;7:254-258. [PubMed] [Cited in This Article: ] |
| 33. | Yip SP. Single-tube multiplex PCR-SSCP analysis distinguishes 7 common ABO alleles and readily identifies new alleles. Blood. 2000;95:1487-1492. [PubMed] [Cited in This Article: ] |
| 34. | Eng C, Brody LC, Wagner TM, Devilee P, Vijg J, Szabo C, Tavtigian SV, Nathanson KL, Ostrander E, Frank TS. Interpreting epidemiological research: blinded comparison of methods used to estimate the prevalence of inherited mutations in BRCA1. J Med Genet. 2001;38:824-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Pitcher D, Sillis M, Robertson JA. Simple method for determining biovar and serovar types of Ureaplasma urealyticum clinical isolates using PCR-single-strand conformation polymorphism analysis. J Clin Microbiol. 2001;39:1840-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Emeny RT, Herron JR, Xi LF, Koutsky LA, Kiviat NB, Wheeler CM. Comparison of variant-specific hybridization and single-strand conformational polymorphism methods for detection of mixed human papillomavirus type 16 variant infections. J Clin Microbiol. 1999;37:3627-3633. [PubMed] [Cited in This Article: ] |
| 37. | Gillman LM, Gunton J, Turenne CY, Wolfe J, Kabani AM. Identification of Mycobacterium species by multiple-fluorescence PCR-single-strand conformation polymorphism analysis of the 16S rRNA gene. J Clin Microbiol. 2001;39:3085-3091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Hall JS, French R, Morris TJ, Stenger DC. Structure and temporal dynamics of populations within wheat streak mosaic virus isolates. J Virol. 2001;75:10231-10243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Baranyay F, Bogár G, Sebestyén M. [Adult Hirschsprung's disease with mental retardation and microcephaly]. Orv Hetil. 2000;141:1673-1676. [PubMed] [Cited in This Article: ] |
| 41. | Koletzko S, Jesch I, Faus-Kebetaler T, Briner J, Meier-Ruge W, Müntefering H, Coerdt W, Wessel L, Keller KM, Nützenadel W. Rectal biopsy for diagnosis of intestinal neuronal dysplasia in children: a prospective multicentre study on interobserver variation and clinical outcome. Gut. 1999;44:853-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Ludman L, Spitz L, Tsuji H, Pierro A. Hirschsprung's disease: functional and psychological follow up comparing total colonic and rectosigmoid aganglionosis. Arch Dis Child. 2002;86:348-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |