Copyright
©The Author(s) 2002.
World J Gastroenterol. Jun 15, 2002; 8(3): 499-504
Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.499
Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.499
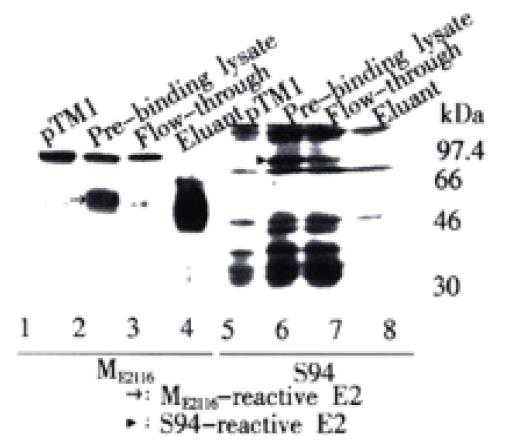
Figure 5 Different ability of differently glycosylated E2 speicies to bind to GNA-agarose.
HeLa cells infected with vTT7 and transfected with pCEH-2 (1-730) were collected, washed and lysed with lysis buffer. The cleared supernatant was then allowed to bind to GNA-agarose. The gel beads were washed and eluted with 1M α-D-mannopyranoside in lysis buffer. Pre-binding lysate, flow-through and eluant fractions were analyzed by Western blot analysis. HeLa cells infected with vTT7 and transfected with pTM1 were served as negative control. The sera used as primary antibodies are indicated at the bottom of the lanes. E2 proteins are indicated by arrowheads.
- Citation: Zhu LX, Liu J, Li YC, Kong YY, Staib C, Sutter G, Wang Y, Li GD. Full-length core sequence dependent complex-type glycosylation of hepatitis C virus E2 glycoprotein. World J Gastroenterol 2002; 8(3): 499-504
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.499