Published online Aug 15, 2001. doi: 10.3748/wjg.v7.i4.500
Revised: January 8, 2001
Accepted: January 15, 2001
Published online: August 15, 2001
AIM: To establish the role of vascular endothelial growth factor (VEGF) in the oncogenesis of human gastric carcinoma more directly.
METHODS: The expression of VEGF and its receptor kinase-domain insert containing receptor (KDR) in human gastric cancer tissue were observed by immunohistochemical staining. VEGF levels were manipulated in human gastric cancer cell using eukaryotic expression constructs designed to express the complete VEGF165 complimentary DNA in either the sense or antisense orientation. The biological changes of the cells were observed in which VEGF was up-regulated or down-regulated.
RESULTS: VEGF-positive rate was 50%, and VEGF was mainly localized in the cytoplasm and membrane of the tumor cells, while KDR was mainly located in the membrane of vascular endothelial cells in gastric cancer tissues and peri-cancerous tissue. In 2 cases of 50 specimens, the gastric cancer cells expressed KDR, localized in both the cytoplasm and membrane. Introduction of VEGF165 antisense into human gastric cancer cells (SGC-7901, immunofluorescence intensity, 31.6%)) resulted in a significant reduction in VEGF-specific messenger RNA and total and cell surface VEGF protein ( immunofluorescence intensity, 8.9%) (P < 0.05). Conversely, stable integration of VEGF165 in the sense orientation resulted in an increase in cellular and cell surface VEGF (immunofluorescence intensity, 75.4%) (P < 0.05). Lowered VEGF levels were associated with a marked decrease in the growth of nude mouse xenografted tumor (at 33 d postimplantation, tomor volume: 345.40 ± 136.31 mm3) (P < 0.05 vs control SGC-7901 group: 1534.40 ± 362.88 mm3), whereas up regulation of VEGF resulted in increased xenografted tumor size (at 33 d postimplantation, tomor volume: 2350.50 ± 637.70 mm3) (P < 0.05 vs control SGC-7901 group).
CONCLUSION: This study provides direct evidence that VEGF plays an important role in the oncogenesis of human gastric cancer.
- Citation: Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, Jin M. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol 2001; 7(4): 500-505
- URL: https://www.wjgnet.com/1007-9327/full/v7/i4/500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i4.500
It is known that malignant tumors depend on neovascularization for their growth and metastasis[1,2]. Recently, many studies have shown that the secretion and activation of various endothelial growth factors, called angiogenic factors, by tumor cells plays a crucial role in the formation of neovasculature[3-8]. VEGF is a powerful mitogen for vascular endothelial cells, both in vitro and in vivo. In addition, VEGF has the property of inducing vascular permeability in vivo. Three evidences for the central role played by VEGF in tumor angiogenesis were: ① the detection of high levels of VEGF expression in palisading cells around regions of necrosis in a number of solid tumor systems, coupled with the rapid induction of VEGF when tumor cells are grown under hypoxic conditions[9,10]; ② injection of antibodies against VEGF markedly reduced the in vivo growth of s.c. injected tumor cells, which are known to produce robust tumor angiogenesis[11]; and ③ introduction into tumor endothelial cells of a dominant-negative version of VEGF-R2 by means of retroviral transfer markedly reduced tumor size[12]. The current study was designed to establish the role of VEGF in the oncogenesis of human gastric cancer more directly.
Resected specimens from 50 patients with gastric carcinoma who underwent gastrectomy at our institute were studied. The patients ranged in age from 27 to 76 years (average 56.3 years); 40 were men, and 10 were women. No patient had received chemotherapy or radiation therapy before surgery. Specimens were fixed in a 40 g·L-1 formaldehyde solution and embedded in paraffin. Five μm thick sections were cut and mounted on glass slides. Immunohistochemistry was performed using the avidin-biotin complex method. Sections were dewaxed in xylene, taken through ethanol, and then incubated with 30 mL·L-1 hydrogen peroxide in methanol for 30 minutes to block endogenous peroxidase activity. Sections were then washed in phosphate-buffered saline and incubated in 20 mL·L-1 normal goat serum for 30 minutes to reduce nonspecific antibody binding. The antibody for VEGF and KDR (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) were used respectively. Specimens were then incubated with a 1:500 dilution of anti-VEGF antibody overnight at 4 °C, followed by three washes with PBS. Sections were then incubated with biotinylated goat antirabbit immunoglobulin G (Sino-American Biotechnology Co.) at a dilution of 1:100 for 2 hours followed by 3 washes. Slides were then treated with the complex of Reagent A and Reagent B (ABC kit, Sino-American Biotechnology Co.) for 2 hours at a dilution of 1: 100 and were washed with phosphate-buffered saline 3 times. Finally, slides were incubated in phosphate-buffered saline containing diaminobenzidine and 300 mL·L-1 hydrogen peroxide for 10 minutes. Normal rabbit immunoglobulin-G was substituted for primary antibody as the negative control.
pGEM-hVEGF is pGEM-3Zf (+) derivative Promega cloning vector plasmid containing the complete complementary DNA (cDNA) sequence of human VEGF165 (this plasmid was generous gifts from Judith Abraham, Scios Nova, Inc.)[13]. The VEGF165 cDNA was subcloned into the pCDNA3 eukaryotic expression vector using the Eco-RI and Xba I or EcoR I and Hind III restriction enzyme sites in sense or antisense orientation respectively. Restriction enzyme analysis (EcoR I, Xba I and Hind III) and dideoxy sequencing method were used to confirm the orientation and quality of the VEGF cDNA in the pCDNA3 vector, respectively.
Cell line SGC-7901 was derived from a moderately-differentiated gastric adenocarcinoma and has been characterized extensively[14]. The cells were routinely cultured in RPMI 1640/100 mL·L-1 NCS (heat-inactivated) and 2 mmol·L-1 L-glutamine in a humidified atmosphere of 50 mL·L-1 CO2 at 37 °C, supplemented with penicillin (100 KU·L-1) and streptomycin (100 mg·L-1).
Human SGC-7901 cells were grown in RPMI 1640/100 mL·L-1 NCS in 6-well tissue culture dishes to 50% confluence. The lipofect Amine-mediated transfection (Gibco/BRL) was performed as described previously using 2 μg of recombinant constructs DNA (or pCDNA3 vector alone)[15]. After 48 h, they were trypsinized from the plates and dilute cells into selective medium containing Geneticin G418 (350 mg·L-1). Cell death was observed after 3 d in culture, and discrete colonies were apparent by 10 d post-selection. Individual colonies were then isolated and grown in 24-well culture plates. Genomic DNA and total RNA were then isolated from these colonies, and PCR analysis and RNA dot blotting were performed. Clones demonstrated to express the sense and antisense-VEGF constructs were then recloned by growing single cell in 96-well plates.
PCR was used to determine which human gastric cancer cell clones were successfully transfected with the sense or antisense-VEGF construct. PCR was performed on genomic DNA isolated from human SGC-7901 gastric cancer cells and individual clones of transfected cells using a sense primer (a) that corresponds to the 5’ initial sequence of VEGF cDNA insert (5’GCACCCATGGCAGAAGGAGGAG3’) or an antisense primer (b) that corresponds to the 3’ terminal sequence (5’TCACCGCCTCGGCTTGTCACATC3’) and a primer that corresponds to the SP6 transcription start of the pCDNA3 expression vector (5’GATTTAGGTGACACTATAG3’). The PCR reaction was performed using standard protocols with 30 cycles of 50 s at 94 °C, 50 s at 55 °C and 60 s at 72 °C. Appropriately sized amplification products were verified by agarose gel electrophoresis. Negative controls lacking target DNA or containing a nonhomologous plasmid routinely did not show amplification.
Total RNA was isolated from parental and derivative cell lines using the Trizol reagent (GIBCO/BRL ). The samples were serially diluted in diethyl pyrocarbonate-treated water containing placental RNase inhibitor (Boehringer Mannheim) to produce working stocks with a final concentration of 10 mg·L-1 as spectrophotometrically. Denatured RNA was immobilized on nitrocellulose membranes (Boehringer Mannheim). “Run-off” digoxigenin (DIG) labeled riboprobes specific for the sense or antisense strands of VEGF was synthesized from appropriately linearized plasmid stocks using the DIG-RNA labeling kit (Boehinger Mannheim) and appropriate RNA polymerase (Promega) plus 10 U placental RNase inhibitor. The specificity of each probe stock was verified by hybridization to originating plasmids and negative controls. The blots were prehybridized in DIG easy Hyb buffer (Boehringer Mannheim), then hybridized for 18 hours at 60 °C in buffer containing 100 μg·L-1 of sense or antisense-specific DIG-labeled probe. After hybridization, the blots were washed extensively and the extent of hybridization was visualized colorimetrically using reagents from the DIG nucleic acid detection kit, Boehringer Mannheim).
Confluent monolayers of parental and derivative cell lines were harvested in calcium and magnesium free Dulbeco’s phosphate-buffered saline (CMF) supplemented with 2 mmol·L-1 EDTA. These stocks were pelleted by centrifugation, resuspended in ice cold CMF with 10 g·L-1 bovine serum albumin and 0.2 g·L-1 sodium azide, quantitated for viability by trypan blue dye exclusion, and then diluted to a final total viable cells concentration of 1 × 109·L-1. Each stock was then exposed to anti-VEGF for 1 hour at 4 °C. The cells were washed three times with ice-cold CMF with 10 g·L-1 bovine serum albumin, then exposed to a 1:20 dilution of fluorescein isothiocyanate- conjugated goat anti-rabbit IgG (Boehringer Mannheim) for 30 minutes at 4 °C. The cells were washed twice in CMF, and extent of surface fluorescence for equal number subpopulations of cells was analyzed by fluorescence-activated cell-sorting (FACSTAR; Becton Dickinson) analysis.
Human gastric cancer cells SGC-7901 and its transfected ones with sense or antisense VEGF were cultured at 4 × 104 cells and grown under standard culture conditions. Cell counts were performed on a hemocytometer, initially at 12 h time points, and then every 24 h for a total of at least 140 h. The total number of cells from duplicate experiments was determined as a function of time (h), and the rate of division was calculated from the exponential phase of growth.
Adult male or female (nu/nu) mice (5-8 weeks of age) received s.c. injections of SGC-7901 gastric cancer cells or SGC-7901 gastric cancer cells transfected with the sense-VEGF or antisense-VEGF constructs. Approximately 106 cells resuspended in a volume of 100 μL of serum free cell culture medium were s.c. injected into the dorsa of mice. The mice were monitored daily, and tumor sizes were determined by tridimensional calliper measurements. Tumor volume was calculated as 1/2 × length × width 2 (mm3). Mean tumor volumes were calculated from measurements performed on five mice in each of two individual experiments. The animals were killed 33 d later. s.c. tumors were removed from mice, formaldehyde fixed, and paraffin-embedded. Five μm thick sections were cut and mounted on glass slides. The sections were stained with hematoxylin and eosin[16-18].
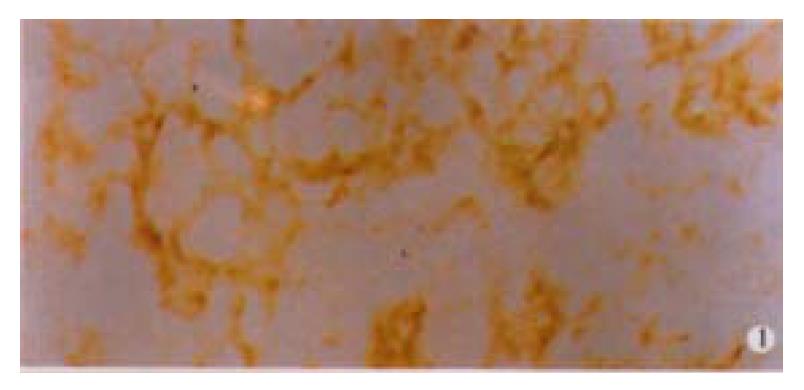
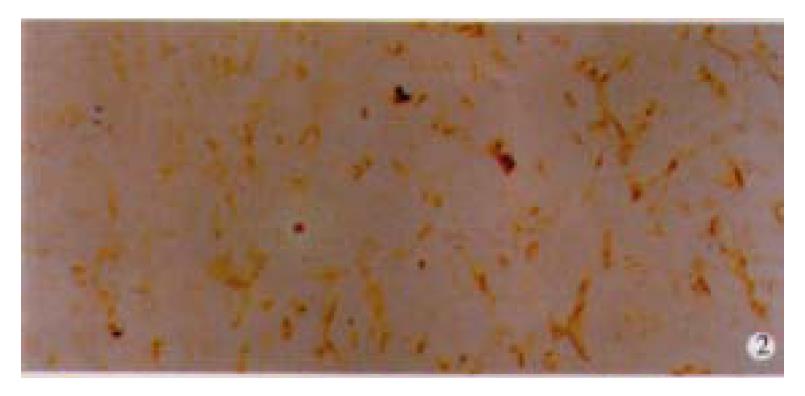
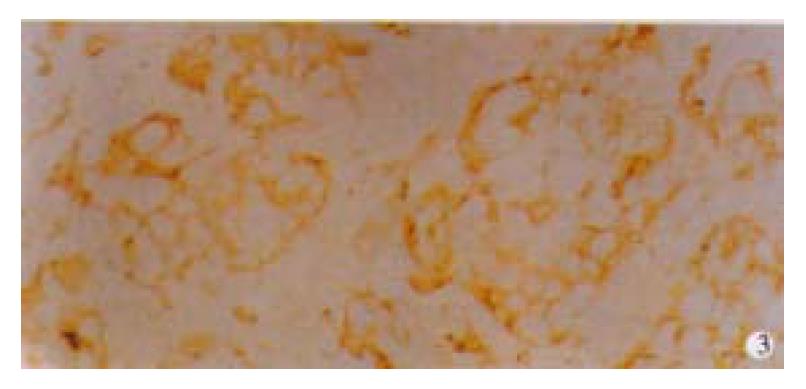
Among 50 formalin-fixed, paraffin-embedded surgically resected tissue specimens of gastric carcinoma, 10 specimens were composed of two different histological type cancer cells, most of which were poorly differentiated adenocarcinoma and mucinous cell carcinoma. Normal gastric mucosa was not immunoreactive with an anti-VEGF antibody. VEGF was mainly localized to the cytoplasm or the membrane of the carcinoma (Figure 1). Tumor cells that stained strongly for VEGF were observed more often in the invasive front than in the tumor center. Weakly positive VEGF staining was seen on some endothelial cells. VEGF expression was detected in 25 (50%) tumors. KDR was mainly localized in the cytoplasm or the membrane of vascular endothelial cells in gastric cancer tissue and peri-cancerous tissue (Figure 2). In 2 cases of 50 specimens, the gastric cancer cells expressed KDR, localized in the cytoplasm and membrane (Figure 3).
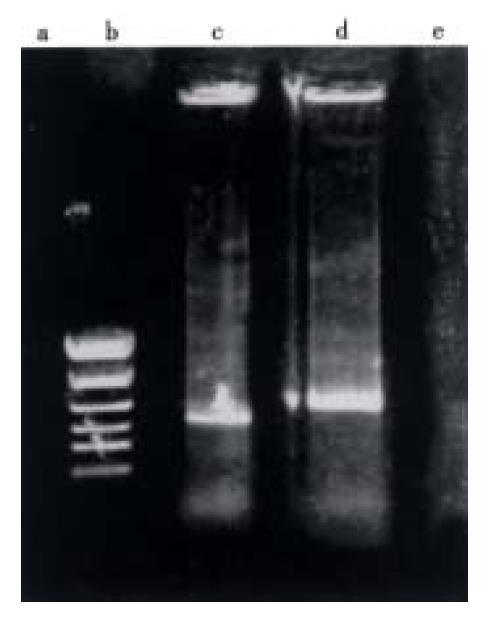
The VEGF165 insert was cleaved from pGEM-3Zf (+) by restriction enzymes digestion and cloned into the same restriction sites of the eukaryotic expression vector, pCDNA3. Restriction enzyme mapping and dideoxy sequencing assay performed on DNA from transformed clones demonstrated the presence of the VEGF165 cDNA insert cloned in the sense and antisense orientation in the pCDNA3 vector (Figure 4).
Following transfection of human SGC-7901 gastric cancer cells with the sense or antisense VEGF construct (or vector alone control) and subsequent antibiotic selection, individual clones were isolated and grown in 24-well culture plates. Polymerase chain reaction (PCR) analysis of DNA isolated from these clones revealed that the positive clones had the sense or antisense VEGF construct. A selection of these clones were then re-cloned at the level of one cell/well, and the PCR analysis was repeated to confirm expression of the cDNA.
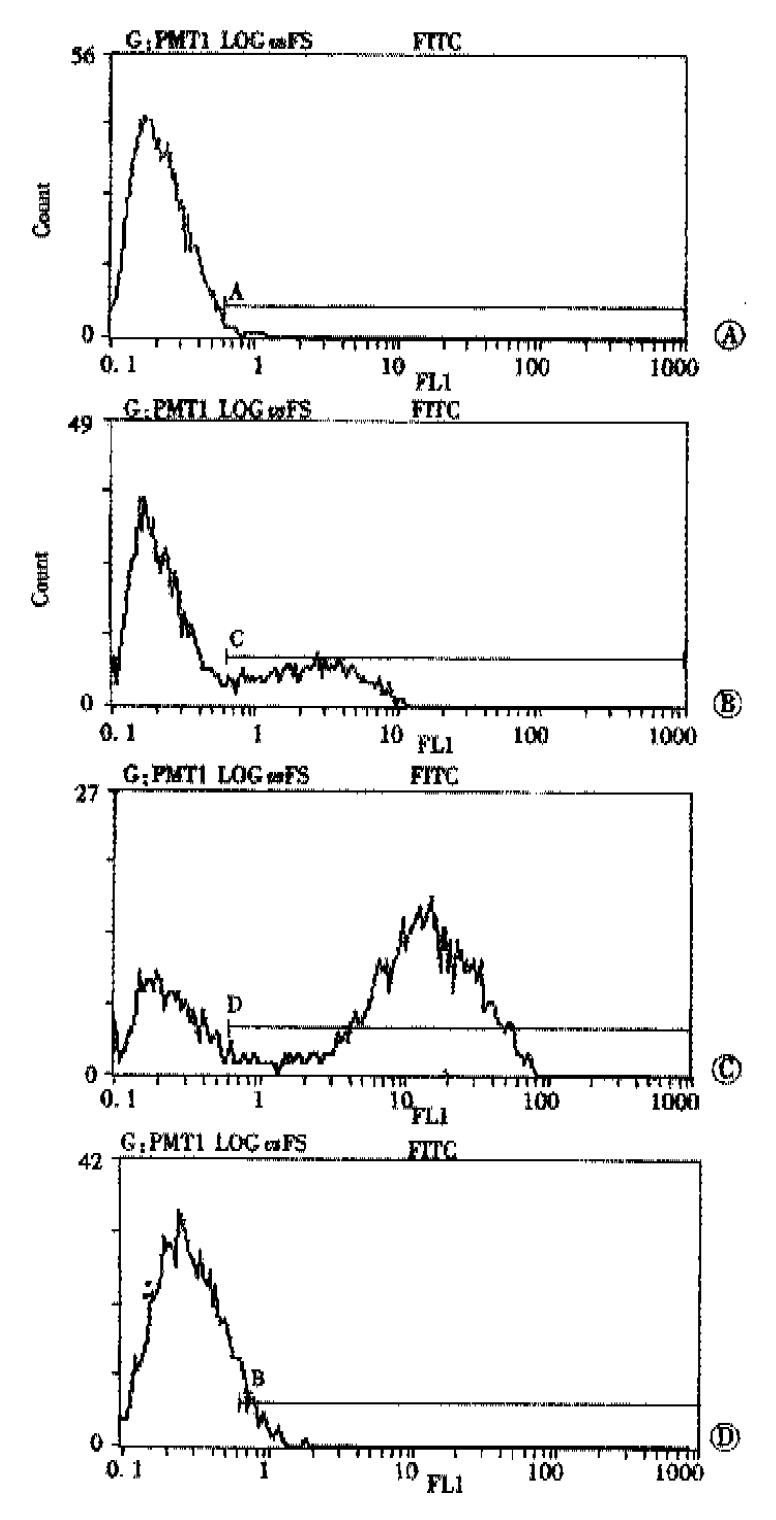
To better determine whether VEGF plays a functional role in the oncogenesis of gastric cancer, gastric cancer cells were transfected with sense or antisense VEGF expression vector, and the effect on the growth of tumor was evaluated. Stable integration of expression constructs into G418-resistant subclones was shown by PCR-based approach that utilized SP6 and VEGF-specific primer pairs and genomic DNA (see Methods). Introduction of the VEGF antisense construct into SGC-7901 cells resulted in a markedly reduction in the expression of VEGF-specific mRNA by dot blot analysis in SGC-7901/as hVEGF compared with the parental cell line. Conversely, the expression of VEGF in mRNA level was enhanced in SGC-7901 cells transfected with the sense-VEGF construct. Alterations in total cellular VEGF after transfection with sense or antisense constructs were accompanied by similar changes in cell surface VEGF protein as determined by flow cytometer analysis (Figure 5).
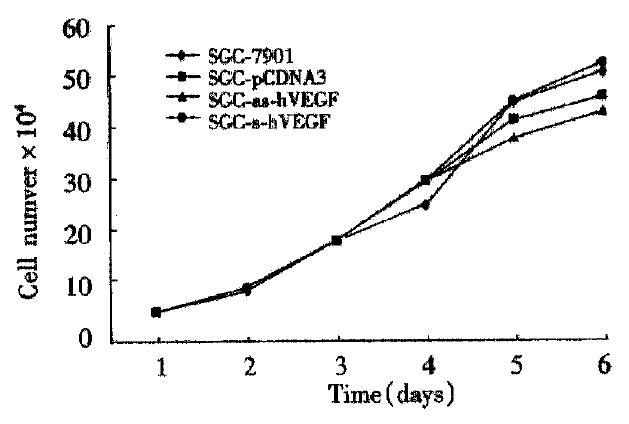
The sense-VEGF cell lines and antisense-VEGF cell lines appeared phenotypically indistinguishable from normal SGC-7901 gastric cancer cells and SGC-7901 transfected vector alone cells, and the growth rates of the derivative cell lines were identical to that of normal SGC-7901 cells (Figure 6).
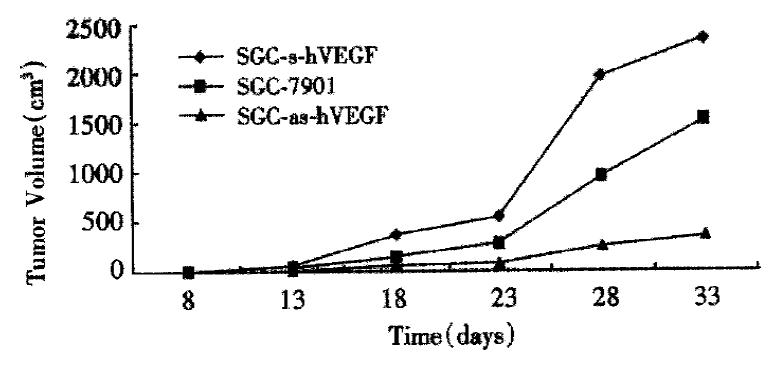
Control human SGC-7901 gastric cancer cell, sense and antisense-VEGF cell lines were s.c. injected into nude mice, and tumor volumes were measured daily for the duration of the experiments. Tumor growth was detectable and measurable by 8 d postimplantation. At this time point, the parental cells, sense-VEGF cells, and antisense-VEGF cells had produced tumors of 13.25 ± 3.58 mm3, 13.46 ± 6.04 mm3 and 12.46 ± 3.01 mm3. There were no significant differences between their volumes(P > 0.05). After 18 d postimplantation, however, the tumors from the sense-VEGF cell lines begun to grow more quickly than that from the parental cells, but the growth rate of tumors from the antisense-VEGF cell lines become slow. At 33 d postimplantation, sense-VEGF, SGC-7901, and antisense-VEGF cell lines produced tumors of 2350.50 ± 637.70 mm3, 1534.40 ± 362.88 mm3, and 345.40 ± 136.31 mm3, respectively (Figure 7). At this time point, the tumor inhibiting rate was 77% through antisense inhibition. Sections of tumors were observed for their degree of tissue necrosis and vascularization. The results demonstrated that there was higher degree of necrosis in the tumors of the antisense-VEGF cell lines in comparison to tumors produced by control SGC-7901 cells, and the number of blood vessels observed in the tumors derived from the sense VEGF cell lines was higher.
Chemotherapy is the main treatment for patients with malignant tumor not to be resected, however, acquired resistance to chemotherapy is a major problem during cancer treatment. One mechanism for drug resistance is overexpression of the MDR (multidrug resistance)1 gene encoding the transmembrane efflux pump, P-glycoprotein (P-gp). In recent years, it has attracted much attention and has been studied as a mechanism of multidrug resistance of tumors to anticancer drugs, however, the application of most agents with the capacity to reverse multidrug resistance (MDR) via modulation of the multidrug transporter P-glycoprotein (Pgp) was shown to be associated with toxic side-effects[19-21]. In addition to it, the heterogenerous of tumor may caused some trouble for choosing sensitive agents. Take altogether, many attempt were made to search for notoxic agents aiming at a common target. Neovascularization is critical for supporting the rapid growth of solid tumors. Tumor angiogenesis appears to be achieved by the expression of angiogenic agents within solid tumors that stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis[22-25]. One such protein, vascular endothelial growth factor, or vascular permeability factor, is a selective endothelial cell mitogen and angiogenic agent induced by several growth factors and cytokines, and elevated expression of either the Ras[26,27], Raf[28], Src[29], or mutant P53[30] oncogenes and hypoxia[31-33] characteristic of rapidly growing solid tumors. VEGF was over expressed in many solid tumors, including breast[34], ovarian[35], lung[36], esophageal[37], and colon[38] cancer. These data suggest a potential role for VEGF in the oncogenesis of solid tumor.
VEGF-positive rate was 50% in human gastric cancer tissues, and it was mainly localized to the cytoplasm and membrane of the tumor cells, while KDR was mainly localized in the endothelial cells. This finding indicated that VEGF might have paracrine effect upon the endothelial cells to promote angiogenesis. In 50 specimens, of 2 cases in which the gastric cancer cells expressed VEGF and its receptor KDR, suggesting that VEGF might have an autocrine effect upon the gastric cancer cells themselves. Analysis of human gastric cancer tissue sections has shown that they are highly heterogeneous[39-41]. The cellular profile of each individual is also heterogeneous in that they contain cells at varying stages of malignancy and have growth factor/receptor expression profile that differ markedly[42-48].
The current study provides a more direct evidence that VEGF plays a role in the growth of gastric cancer. Reduction of VEGF mRNA and protein levels through antisense inhibition significantly lowered the growth rate of the tumors from antisense-VEGF cell lines. Conversely, elevation of VEGF levels after sense transfection resulted in a significant increase of the growth rate of the tumors from sense VEGF cell lines. These results provide strong evidence that VEGF plays an important role in the oncogenesis of the gastric cancer.
A potential therapy based on the interruption of paracrine and/or autocrine growth factor pathways that impinge upon the tumor cells themselves might well prove to be a successful antitumor approach[49-53]. Exploiting the ubiquity of tumor angiogenesis as a suitable target for therapy has been proposed previously to be an important concept for antitumor therapy[54]. This concept has recently been the subject of renewed interest in the development of new therapeutic strategies. Significant evidences are accumulating in favor of the notion that VEGF and its receptor play important roles in the development of solid tumors, such as those derived from gastric cancer origin.
Much indirect evidences indicate that VEGF is an important participant in tumor biology[11,12,55,56]. We now provide direct evidence that VEGF plays an important role in the growth of gastric cancer. VEGF might have mainly paracrine effect upon the endothelial cells to promote angiogenesis[57-62], and it might have an autocrine effect upon the gastric cancer cells themselves. The inhibition of VEGF is sufficient to control tumor growth in vivo by the suppression of tumor neovascularization[63-69]. The antisense VEGF strategy offers a new avenue of gene therapy development as an adjuvant treatment for human gastric cancer[70-76].
Edited by Ma JY
| 1. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3254] [Cited by in F6Publishing: 3164] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 2. | Bicknell R, Harris AL. Novel growth regulatory factors and tumour angiogenesis. Eur J Cancer. 1991;27:781-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 118] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3324] [Cited by in F6Publishing: 3168] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 4. | Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297-7301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 544] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W, Heldin CH. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 510] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Tao HQ, Lin YZ, Wang RN. Significance of vascular endothelial growth factor messenger RNA expression in gastric cancer. World J Gastroenterol. 1998;4:10-13. [PubMed] [Cited in This Article: ] |
| 7. | Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296-300. [PubMed] [Cited in This Article: ] |
| 8. | Xue JT, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF(121) in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6:281-283. [PubMed] [Cited in This Article: ] |
| 9. | Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1572] [Cited by in F6Publishing: 1568] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3206] [Cited by in F6Publishing: 3175] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 11. | Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2567] [Cited by in F6Publishing: 2501] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 12. | Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 857] [Cited by in F6Publishing: 926] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 13. | Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947-11954. [PubMed] [Cited in This Article: ] |
| 14. | Lin CH, Fu ZM, Liu YL, Yang JL, Xu JF, Chen QS, Chen HM. Investigation of SGC-7901 cell line established from human gastric carcinoma cells. Chin Med J (Engl). 1984;97:831-834. [PubMed] [Cited in This Article: ] |
| 15. | Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413-7417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3313] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 16. | Inaba M, Tashiro T, Kobayashi T, Fujimoto S, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T. Evaluation of response rates to various antitumor agents of human gastric tumors implanted in nude mouse. Jpn J Cancer Res. 1986;77:190-196. [PubMed] [Cited in This Article: ] |
| 17. | Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210-215. [PubMed] [Cited in This Article: ] |
| 18. | Feng GG, Zhou XG, Yu BM. Prevention of metastasis to liver by using 5-FU intraperitoneal chemotherapy in nude mice inoculated with human colonic cancer cells. China Natl J New Gastroenterol. 1996;2:134-135. [Cited in This Article: ] |
| 19. | Liu ZM, Shou NH. Expression significance of mdr1 gene in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145-146. [Cited in This Article: ] |
| 20. | Meng ZQ, Yu EX, Song MZ. Inhibition of telomerase activity of human liver cancer cell SMMC-7721 by chemotherapeutic drugs. Shijie Huaren Xiaohua Zazhi. 1999;7:252-254. [Cited in This Article: ] |
| 21. | Lu XP, Li BJ, Chen SL, Lu B, Jiang NY. Effect of chemotherapy or targeting chemotherapy on apoptosis of colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:332-334. [Cited in This Article: ] |
| 22. | Tao HQ, Qing LF, Lin YZ, Wang RN. Significance of microvessel count in gastric carcinoma. China Natl J New Gastroenterol. 1996;2:86-88. [Cited in This Article: ] |
| 23. | Tao HQ, Qin LF, Lin YZ, Wang RN. Expression of vascular endothelial growth factor and its prognostic significance in gastric carcinoma. China Natl J New Gastroenterol. 1996;2:128-130. [Cited in This Article: ] |
| 24. | Liao XB, Tang WP, Zhang QM, Fu ZG, Zhao YH. Morphological analysis of lymph vessels and capillaries in gastric carcinoma. China Natl J New Gastroenterol. 1997;3:90-92. [Cited in This Article: ] |
| 25. | Li XR, Wu JS, He ZS, Ma QJ, Gao DM. Overproduction of nitric oxide inhibits vascular reactivity in portal hypertensive rats. China Natl J New Gastroenterol. 1997;3:221-224. [Cited in This Article: ] |
| 26. | Diaz-Laviada I, Larrodera P, Diaz-Meco MT, Cornet ME, Guddal PH, Johansen T, Moscat J. Evidence for a role of phosphatidylcholine-hydrolysing phospholipase C in the regulation of protein kinase C by ras and src oncogenes. EMBO J. 1990;9:3907-3912. [PubMed] [Cited in This Article: ] |
| 27. | Kolch W, Heidecker G, Lloyd P, Rapp UR. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349:426-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 391] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Kolch W, Heidecker G, Troppmair J, Yanagihara K, Bassin RH, Rapp UR. Raf revertant cells resist transformation by non-nuclear oncogenes and are deficient in the induction of early response genes by TPA and serum. Oncogene. 1993;8:361-370. [PubMed] [Cited in This Article: ] |
| 29. | Mukhopadhyay D, Tsiokas L, Sukhatme VP. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995;55:6161-6165. [PubMed] [Cited in This Article: ] |
| 30. | Volpert OV, Dameron KM, Bouck N. Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Oncogene. 1997;14:1495-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355-4359. [PubMed] [Cited in This Article: ] |
| 32. | Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 375] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Salceda S, Beck I, Caro J. Absolute requirement of aryl hydrocarbon receptor nuclear translocator protein for gene activation by hypoxia. Arch Biochem Biophys. 1996;334:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 372] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Abu-Jawdeh GM, Faix JD, Niloff J, Tognazzi K, Manseau E, Dvorak HF, Brown LF. Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Lab Invest. 1996;74:1105-1115. [PubMed] [Cited in This Article: ] |
| 36. | Volm M, Koomägi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 37. | Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 38. | Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964-3968. [PubMed] [Cited in This Article: ] |
| 39. | Xu T, Fang LP, Li J, Liu WQ, Zhou ZY, Qiu CP, Yu XH, Si TP, He LJ, Fang XL. A pathologic analysis of 4451 cases with digestive tract cancer. World J Gastroenterol. 1998;4:65-66. [Cited in This Article: ] |
| 40. | Luo YQ, Wu MC, Cong WM. Gene expression of hepatocyte growth factor and its receptor in HCC and nontumorous liver tissues. World J Gastroenterol. 1999;5:119-121. [PubMed] [Cited in This Article: ] |
| 41. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] [Cited in This Article: ] |
| 42. | Wang LD, Zhou Q, Gao SS, Li YX, Yang WC. Measurements of cell prolifera-tion in esophageal and gastric cardia epithelia of subjects in a high incidence area for esophageal cancer. China Natl J New Gastroenterol. 1996;2:82-85. [Cited in This Article: ] |
| 43. | Wang YK, Ji XL, Gu YG, Zhang SC, Xiao JH. P53 and PCNA expression in glandu-lar dilatation of gastric mucosa. China Natl J New Gastroenterol. 1996;2:106-108. [Cited in This Article: ] |
| 44. | Yu JY, D'Adda T. Quantitative ultrastructure analysis of neuroendocrine cells of gastric mucosa in normal and pathological conditions. China Natl J New Gastroenterol. 1996;2:155-157. [Cited in This Article: ] |
| 45. | Yang GL, Dong YM, Du WD, Su YH, Zhang H, Wu JF, Wang DB, Xu AL. Ultra-structural enzyme cytochemical study on signet-ring cells of gastric cancer. China Natl J New Gastroenterol. 1997;3:86. [Cited in This Article: ] |
| 46. | Wang DX, Fang DC, Luo YH, Liu WW. Loss of heterozygosity and mRNA expression at DCC locus in gastric cancer. China Natl J New Gastroenterol. 1997;3:156-159. [Cited in This Article: ] |
| 47. | Wu GJ, Shan XN, Li MF, Shi SL, Zheng QP, Yu L, Zhao SY. A preliminary study on the loss of heterozygosity at 17p13 in gastric and colorectal cancers. China Natl J New Gastroenterol. 1997;3:160-162. [Cited in This Article: ] |
| 48. | Dai YM, Chen H, Wang NJ, Ni CR, Cong WM, Zhang SP. Clinicopathologic risk factors and prognostic evaluation in hepatocellular carcinoma recurrence after surgery. China Natl J New Gastroenterol. 1997;3:199. [Cited in This Article: ] |
| 49. | Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol. 1998;4:30-32. [PubMed] [Cited in This Article: ] |
| 50. | Xu CT, Pan BR. Current status of gene therapy in gastroenterology. World J Gastroenterol. 1998;4:85-89. [PubMed] [Cited in This Article: ] |
| 51. | Schmid R. Prospect of gastroenterology and hepatology in the next century. World J Gastroenterol. 1999;5:185-190. [PubMed] [Cited in This Article: ] |
| 52. | Xiao B, Jing B, Zhang YL, Zhou DY, Zhang WD. Tumor growth inhibition effect of hIL-6 on colon cancer cells transfected with the target gene by retroviral vector. World J Gastroenterol. 2000;6:89-92. [PubMed] [Cited in This Article: ] |
| 53. | Yang DH, Zhang MQ, Du J, Xu C, Liang QM, Mao JF, Qin HR, Fan ZR. Inhibitory effect of IGF- II antisense RNA on malignant phenotype of hepatocellular carcinoma. World J Gastroenterol. 2000;6:266-267. [PubMed] [Cited in This Article: ] |
| 54. | Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 825] [Cited by in F6Publishing: 795] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 55. | Goldman CK, Kendall RL, Cabrera G, Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ, Huckle WR. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc Natl Acad Sci USA. 1998;95:8795-8800. [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 323] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 56. | Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |
| 57. | Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Fidler IJ. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol. 2000;157:1893-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6:4287-4291. [PubMed] [Cited in This Article: ] |
| 59. | Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, Fox SB. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res. 2000;6:4278-4286. [PubMed] [Cited in This Article: ] |
| 60. | Ishibashi H, Nakagawa K, Onimaru M, Castellanous EJ, Kaneda Y, Nakashima Y, Shirasuna K, Sueishi K. Sp1 decoy transfected to carcinoma cells suppresses the expression of vascular endothelial growth factor, transforming growth factor beta1, and tissue factor and also cell growth and invasion activities. Cancer Res. 2000;60:6531-6536. [PubMed] [Cited in This Article: ] |
| 61. | Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res. 2000;60:6253-6258. [PubMed] [Cited in This Article: ] |
| 62. | Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha--& gt; hypoxia response element--& gt; VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248-6252. [PubMed] [Cited in This Article: ] |
| 63. | Yuan A, Yu CJ, Chen WJ, Lin FY, Kuo SH, Luh KT, Yang PC. Correlation of total VEGF mRNA and protein expression with histologic type, tumor angiogenesis, patient survival and timing of relapse in non-small-cell lung cancer. Int J Cancer. 2000;89:475-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 64. | Karth J, Ferrer FA, Perlman E, Hanrahan C, Simons JW, Gearhart JP, Rodriguez R. Coexpression of hypoxia-inducible factor 1-alpha and vascular endothelial growth factor in Wilms' tumor. J Pediatr Surg. 2000;35:1749-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Kang MA, Kim KY, Seol JY, Kim KC, Nam MJ. The growth inhibition of hepatoma by gene transfer of antisense vascular endothelial growth factor. J Gene Med. 2000;2:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 66. | Nguyen JT. Vascular endothelial growth factor as a target for cancer gene therapy. Adv Exp Med Biol. 2000;465:447-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Sasaki M, Wizigmann-Voos S, Risau W, Plate KH. Retrovirus producer cells encoding antisense VEGF prolong survival of rats with intracranial GS9L gliomas. Int J Dev Neurosci. 1999;17:579-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Im SA, Gomez-Manzano C, Fueyo J, Liu TJ, Ke LD, Kim JS, Lee HY, Steck PA, Kyritsis AP, Yung WK. Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59:895-900. [PubMed] [Cited in This Article: ] |
| 69. | Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF. Adeno-associated virus-mediated delivery of antiangiogenic factors as an antitumor strategy. Cancer Res. 1998;58:5673-5677. [PubMed] [Cited in This Article: ] |
| 70. | Oku T, Tjuvajev JG, Miyagawa T, Sasajima T, Joshi A, Joshi R, Finn R, Claffey KP, Blasberg RG. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998;58:4185-4192. [PubMed] [Cited in This Article: ] |
| 71. | Laitinen M, Ylä-Herttuala S. Adventitial gene transfer to arterial wall. Pharmacol Res. 1998;37:251-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Neckers LM, Kanekal M, Connell Y. Non-antisense oligonucleotide approaches for experimental treatment of glioblastoma. Antisense Nucleic Acid Drug Dev. 1998;8:177-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Darius H, Buerke M, Boissel JP, Grosser T, Veit K, Zacharowski K, Meyer J. [Local drug delivery and gene therapy]. Herz. 1997;22:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA. 1996;93:4851-4856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 239] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Saleh M, Stacker SA, Wilks AF. Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence. Cancer Res. 1996;56:393-401. [PubMed] [Cited in This Article: ] |
| 76. | Martiny-Baron G, Marmé D. VEGF-mediated tumour angiogenesis: a new target for cancer therapy. Curr Opin Biotechnol. 1995;6:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |