Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.259
Revised: June 6, 1999
Accepted: June 13, 1999
Published online: April 15, 2000
AIM: To establish a new improved vascular anastomotic technique to simplify the surgical technique and increase the survival rate of small intestinal transplantation in rats.
METHODS: The graft removed enbloc consisted of entire small intestine, portal vein and aortic segment with superior mesenteric artery. The graft was perfused in situ and the gut lumen was irrigated during the operation. Heterotopic small bowel transplantation was performed by microvascular end-to-side anastomosis between the donor aortic segment with superior mesenteric artery and the recipient abdominal aorta, and by the formation of a “Cuff” anastomos is between the donor portal vein and the recipient left renal vein. Both ends of the grafts were exteriorized as stomas.
RESULTS: A total of 189 intestinal transplantations were performed in rats, 33 of which were involved in the formal experimental group, with a survival rate of 84.8%. The average time for the donor surgery was 80 min ± 10 min; for graft repair 10 min ± 3 min; and for recipient surgery 95 min ± 15 min. The average time for the arterial anastomosis and the vein anastomosis was 18 min ± 5 min and 1 min, respectively. The warm ischemic time and cold ischemic time were 22 min ± 5 min and less than 60 min, respectively. The whole operation was completed by a single surgeon, the operative time being about 3 h.
CONCLUSION: The vascular anastomosis used in this study could simplify surgical technique, reduce the operative time and elevate the survival rate of small intestinal transplantation in rats.
- Citation: Li YX, Li JS, Li N. Improved technique of vascular anastomosis for small intestinal transplantation in rats. World J Gastroenterol 2000; 6(2): 259-262
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.259
Small intestinal transplantation may eventually become the most logical definitive treatment for small bowel syndrome. The small intestinal transplantation in rats has been the most frequently used animal model in the experimental investigation since it was first described by Monchik and Russel in 1971[1]. However, the surgical technique of intestinal transplantation is rather complex and difficult, and the vascular anastomosis is a key technique for successful transplantation. We began to explore intestinal transplantation in rats in 1995, and have accumulated some experience and developed a stable and easy vascular anastomosis technique for small intestinal transplantation in rats.
Male outbred Sprague-Dawley rats, inbred F344/N rats and inbred Wistar/A rats weighing between 150 g and 400 g were used as donors and recipients which were selected according to the protocol design. The rats received 5% glucose and 0.9% saline ad libitum for 24 h (donors) and 12 h (recipients) in the metabolic cage. They were anesthetized with intraperitoneal injection of pentobarbital sodium (40 mg/kg) and atropine (0.05 mg/kg). Both donors and recipients received lactated Ringer′s solution at a rate of 4 mL/h via the tail vein during surgery, the injection being controlled by a microdosage transfusion pump. Polyethylene (PE) catheter (4 mm in length) with an inner diameter of 1.5 mm was used to make a cuff, two-thirds of the circumference diameter of the lower half part (2 mm in length) of the PE catheter was cut off to form a holder in order to be grasped, and a shallow slot was cut out in the middle of outer tube wall of the upper half part (2 mm in length) in order to be ligated.
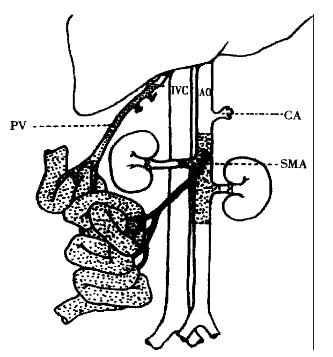
The abdomen was opened via a cross incision. The distal abdominal aorta was ligated beneath the level of renal artery to maintain adequate blood flow to the graft. Left renal vein, both sides of renal arteries and the origin of tuncus coeliacus were mobilized, ligated and divided. The origin of the superior mesenteric artery was mobilized, and the lumbar arteries from the aorta were meticulously ligated with 5-0 silk sutures. A segment of aorta with superior mesenteric artery was thoroughly liberated. The jejunum was divided just below the Treitz′s ligament, the terminal of ileum was separated from the ileocecal valve, and the entire colon was removed. The portal vein and the superior mesenteric vein were met iculously separated from the pancreas and the surrounding conjunctive tissues, the pyloric and splenic veins which refluxed to portal vein were ligated and divided with 7-0 silk suture. Five mL 0.2% amikacin was slowly and gently infused into the intestine from the break-off of the jejunum to irrigate the gut lumen. The proximal end of the prepared aorta segment was ligated, the distal part of the aorta was cannulated with a fine polyethylene, and the graft was perfused in situ with 2 mL-3 mL cold heparinized (25 U/mL) lactated Ringer′s solution at a rate of 40 mL/h controlled by a microdosage transfusion pump. At the same time, the portal vein was separated from the hepatichilus, smashed ice crystal was put on the graft for rapid cooling. When the wall of intestine and mesentery turned white, and when a clear effluent from the portal vein appeared, the intestine and its vascular supply were removed enbloc (Figure 1) and stored in lactated Ringer′s solution at 4 °C. Under condition of 4 °C, the distal end of the aortal segment was ligated, the proximal of the aortal segment was made smooth, the holder of the Cuff was gently fixed with microsurgical vascular forceps, the portal vein was everted and covered onto the outer wall of the cuff, and fixed with ligation with 5-0 silk suture.
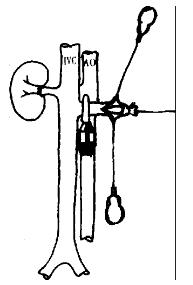
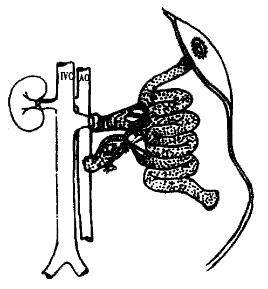
The abdomen was opened via a midline and left abdominal wall horizontal incision was made to form a shape incision. The operative field was exposed with a self-retaining retractor. The left kidney and its surrounding tissues were mobilized. The origin of the left renal artery was ligated, and the tributaries of the left renal vein were ligated and divided. The left renal blood vessel was ligtated at the level of renal hilus. The ligated suture remained counter-retracted, and the left kidney was then removed. Segments (about 1 cm - 1.5 cm long) of the recipient′s abdominal aorta was mobilized below the vessels to the left kidney. The small branches of this segment was carefully ligated. The mobilized segments were controlled promixally and distally with two microvascular clamps, and opened via a longitudinal arteriotomy. The incision of arteriotomy was made to suit the anastomosis of the abdominal aorta of the donor. Heparinized (25 U/mL) normal saline was used to wash away the residual blood clot in the vessel. The graft was placed on the lower left quarter of the abdominal cavity, and was adjusted to ensure that the vessels of donor′s small intestinal were not twisted and had no tension when they were anastomosed. The graft was surrounded by a tampon packed with ice crystals for cooling. The end-to-side anastomosis between the segment of aorta with superior mesenteric artery of the donor and the abdominal aorta of the recipient was performed with the aid of a binocular operating microscope. Two 9.0 nylon stay sutures were placed at both proximal and distal points of the arterial anastomosis to act as self-retaining retractors, and the anastomosis was performed using continuous 9-0 nylon suture. The essentials of the arterial anastomosis were that the interval of the sutures and the edge distance of the anastomosis should be homogenous, and the intimae of the arteries should be everted to form intima-to-intima anastomosis so as to prevent blood clotting. Eighteen to twenty sutures were needed for the anastomosis. When the venous vessels were anastomosed, a microvascular clamp was used to block the origin of the venous vessels of the left kidney. A hemostat was used to counter retract the ligated suture of left kidney hilum to fully unfold the renal venous vessel. A “T” shape incision was made on the anterior wall of the distal segment of left renal vein, and two angles of the incision was counter retracted with 9-0 nylon suture (Figure 2). Heparinized (25 U/mL) normal saline was used to flush the venous vessel, the position of graft was adjusted. After ensuring that the venous vessel was not twisted, the Cuff of the portal vein of the donor was inserted in the left venous vessel of the recipient, and a ligation of 5-0 suture was used to fix the Cuff anastomosis (Figure 3). After the venous clamp was released, followed by the distal and proximal arterial clamps, pulsation of the superior mesentery artery of the graft turned to be distinguished, and the wall of the transplanted intestine appeared fresh red, and the ice-cream intestinal juice secreted from the intestinal lumen. Both ends of the graft were exteriorized as stomas. After flushing the abdominal lumen with normal saline containing cefazolin (10 g/L) of 37 °C, the abdominal wall was closed.
The recipients were under meticulous observation after operation. Autopsy was performed if the recipient died, and the graft, the anastomosis of the vessels, heart, lung, liver, spleen, kidney and the native intestine of the dead recipient were harvested for pathological examination to find out the cause of death. Any death within 5 d was considered technical failure, and survival exceeding 5 d was considered technical success.
Our study was carried out in 3 stages and 189 operations were performed. Thirty-two operations were made to familiarize the anatomy of rats and train the surgical skill in the first stage. The second stage was the pre-experiment, 124 operations were performed to set up the surgical procedure, which was standardized and routinized, the longest survival of the recipient being longer than 10 mo. The third stage was formal experiment, 28 cases of 33 operations succeeded. The cause of failure in the remaining 5 cases was anaesthesia accident (1), thrombosis of the arterial anastomosis (1), massive haemorrhage in abdominal lumen on postoperative day 3 (1), and hypovoluemic shock followed by massive ha emorrhage during operation (2). The success rate of the formal experiments was 84.8%.
The average time for the donor surgery was 80 min ± 10 min; the preparation time for the graft was 10 min ± 3 min; the operative time for the recipient was 95 min ± 15 min, and the time for arterial anastomosis and the venous anastomosis being 18 min ± 5 min and 1 min, respectively. The warm ischemic time of the graft (the interval when the graft was taken away from low temperature preservation) was 22 min ± 5 min, and the cold ischemic time was limited within 60 min. The entire surgery was completed by a single surgeon, and the total operative time being about 3 h.
There are five anastomosis patterns for small intestinal transplantation in rats: ① end-to-side arterial anastomosis between a segment of abdominal aorta/superior mesenteric artery of the donor and abdominal aorta of the recipient, and end-to-side venous anastomosis between the portal vein of the donor and inferior venacava of the recipient[2,3]. This is a classical pattern, and adopted by most investigators. ② End-to-side anastomosis between the carrel′s patch of abdominal aorta with superior mesenteric artery of the donor and the abdominal aorta of the recipient, end-to-side anastomosis between the portal vein of the donor and the portal vein of the recipient[4]. Compared with the cava-refluxing pattern, this portal-vein-refluxing pattern accords with physiology, and delays occurrence of rejection. However, this surgical technique is more difficult, the incidence of complication is rather high, and a segment of the intestine of the recipient should be removed. ③ End-to-side vascular vessels a nastomosis between the superior mesenteric artery of the donor and the abdominal aorta of the recipient, and between the superior mesenteric vein of the donor and the inferior venacava of the recipient[5]. The main advantage of this model is that the donor operation is simplified by obviating the tedious dissection of portal vein and aorta which are very easily injured. However, a limitation of this pattern is that only a 10 cm-25 cm segment of jejunum can be used for transplantation rather than the total small bowel, and the small lumen of peripheral superior mesenteric artery and superior mesenteric vein may increase the vascular complication[6]. Gordon compared pattern 1 with pattern 3 and discovered that there was no significant difference between these two patterns in the operation time, technic complexity and the experimental result[7]. ④ The left native kidney is removed and revascularization of the graft is completed by two end-to-end anastomoses between the donor superior mesenteric artery and portal vein and the recipient renal vessels with the Cuff technique[8]. The advantage of this pattern is that the technique for vascular anastomoses is simple, and has significantly reduced the graft warm ischemic time. However, the graft can only get 33% of the volume of blood supply that the native intestine can obtain, and the graft may lead to dysfunction because of chronic ischemia[7]. ⑤ End-to-end anastomoses between the donor superior mesenteric vessels and the recipient superior mesenteric vessels, respectively. In this pattern, only a segment intestine is transplanted, and a segment of the native intestine should be removed. Furthermore, the incidence of vascular complication is very high[9].
In our study, we combined the advantage of pattern 1 and pattern 4, and adopted arterial anastomosis by microvascular end-to-side anastomosis between the donor aortic segment with superior mesenteric artery and the recipient abdominal aorta, and performed the vein anastomosis by the formation of a “Cuff” anastomosis between the donor portal vein and the recipient left renal vein. This pattern can avoid hemodynamic unstability of the recipient because there is no need to block the inferior vena cava of the recipient while there is a need in pattern 1. At the same time, it can avoid performing anastomoses of both the artery and vein vessels on the adjacent location of the abdominal aorta and the inferior vena cava of the recipient. Thus, exposure of arterial anastomosis is more clear, making the arterial anastomosis very easy. In the arterial end-to-side anastomosis between a segment of the abdominal aorta of the donor and abdominal aorta of the recipient, the caliber of the anastomotic vessels is large enough to make the graft get sufficient blood supply. Therefore, this pattern can avoid chronic ischemia of the graft because of the end-to-end anastomosis between the superior mesenteric artery of the donor and the left renal artery of the recipient in pattern 4. The venous anastomosis with the Cuff technique can be accomplished within 1 min, and the time for venous anastomosis is much shorter than that for traditional venous anastomosis. Thus, the warm ischemic and operation time is reduced, the surgical strike of the recipient is decreased, and the warm ischemic injury of the graft is reduced. The leakage of venous anastomosis is not taken into account after reperfusion because of the Cuff venous anastomosis technique, and no venous thrombus occurs after transplantion because the Cuff venous anastomosis is an intima-to-intima anastomosis of the vessel, and venous thrombus is one of the commonest causes of technique failure in traditonal venous anastomosis. In summary, our improved vascular anastomosis technique, in which the arterial anastomosis is adopted by microvascular end-to-side anastomosis between the donor aortic segment with superior mesenteric artery and the recipient abdominal aorta, and the venous anastomosis is performed by the formation of a “Cuff” anastomosis between the donor portal vein and the recipient left renal vein, could simplify the surgical process, reduce the operation time and increase the survival rate of small intestinal transplantation in rats.
Edited by Ma JY
| 1. | Sonnino RE. Experimental basis for intestinal transplantation. Transplant Proc. 1997;29:1795-1797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693-702. [PubMed] [Cited in This Article: ] |
| 3. | Zhong R, Grant D, Sutherland F, Wang PZ, Chen HF, Lo S, Stiller C, Duff J. Refined technique for intestinal transplantation in the rat. Microsurgery. 1991;12:268-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Kort WJ, Westbroek DL, MacDicken I, Lameijer LD. Orthotopic total small bowel transplantation in the rat. Eur Surg Res. 1973;5:81-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Harmel RP. A simplified technique of small intestinal transplantation in the rat. J Pediatr Surg. 1984;19:400-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sonnino RE, Harmel RP. Technical aspects of intestinal transplantation in the rat. J Invest Surg. 1988;1:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Gordon AC, Dallman MJ, Morris PJ. Vascular anastomotic techniques for experimental intestinal transplantation. Transpl Int. 1994;7:368-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Wallander J, Holtz A, Larsson E, Gerdin B, Läc kgren G, Tufveson G. Small-bowel transplantation in the rat with a nonsuture cuff technique. Technical and immunological considerations. Transpl Int. 1988;1:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lui T. Experimental and clinical study of small intestinal transplantation. Doctorate′s Thesis of Tianjin Medical University. 1997;10-19. [Cited in This Article: ] |