TEXT
Gastrointestinal radiography (GIR) has been a major and first-choice method for diagnosing gastrointestinal diseases with barium as contrast media since its emergence in 1910, even in diagnosing the mass lesions of the organs outside gastrointestinal tract (e.g., liver, pancreas, etc) indirectly. The fiber endoscopy invented in the late 1960s can directly observe the changes on mucosal surface intraluminally and obtain biopsies as well, thus greatly improving the detection and sensitivity of small, shallow or tiny lesions originated from mucosa. This discovery is a big challenge to GIR, which has not only profoundly modified the dominant role of GIR in diagnosing gastrointestinal diseases, but also aroused different viewpoints and evaluations even more radically[1]. According to the data collected from 69 radiologic units during the 12 years by the American Society of Gastrointestinal Radiography, the number of upper GIR decreased by 24%, colon examinations decreased by 29%, averaging 25%[2]. The situation is similar domestically. Some GI radiologists even gave up their experienced studies in GIR. Young radiologists are just eager to study the new inventions such as CT and MR, and neglect GI. All those interfered with and even lowered the quality and quantity of GIR. This is one aspect of the problem.
On the other hand, the invention of endoscopy has positive influence on GIR. It has made many GI radiologists realize that lots of diseases can not be detected with the single contrast GIR, which can not meet the demand of therapy and must be improved. In the 1960s, Japanese scholars developed and created the brand new double-contrast GI technique on the basis of the traditional single-contrast GIR[3], meanwhile, through studies on barium contrast media, assistant agents (mucus detergent, gas agent)[4,5], radiologic technique, the methods and the principles of imaging[6,7], great achievements in practice and theory have been made (Figure 1)[8]. The double-contrast GIR became the universal method in the 1980s, the sensitivity of double-contrast GIR in diagnosing early cancer[9,10], superficial erosion and linear ucler become higher and higher[11]. It made it possible to observe the locations such as cardia which can be hardly shown by single-contrast GIR much easier for diagnosis[12,13], thus re-establishing the status of GIR with high prestige. But it is a regret that this achievement could not be popularized nationwide.
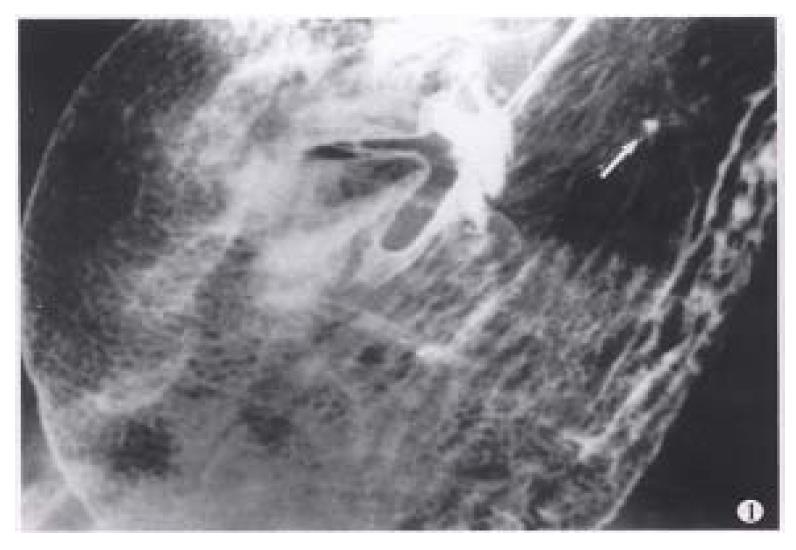
Figure 1 Areae gastricae.
Well-circumscribed polygonal radiolucencies surrounded by barium-filled shallow grooves are clearly seen on the mucosal surface. A small benign ulcer is also displayed (arrowhead).
The multiphase gastrointestinal radiography (MPGIR) can not only diagnose shallow mucosal lesions as endoscopy, but also has its own special effects that other approaches can not possess in diagnosing gastrointestinal diseases[14].
AN IDEAL FIRST-CHOICE DIAGNOSTIC MODALITY FOR GI TRACT
It has been well known that the different diseases of gastrointestinal tract usually present the similar symptoms, which are atypical. Clinical doctors can hardly ascertain the exact location of the lesions when making the diagnoses, and the physical diagnostic approaches are usually necessary. The symptoms caused by these lesions include the following aspects: most of them originated from the mucosa of the gastrointestinal tract, such as inflammation, erosion, ulcer and cancer, etc. (Figure 2); some originated from the submucosa, such as nonepithelial (e.g. smooth muscle, fat and nerve) benign or malignant tumors[15]; some are functional disorders[16], such as reflux disorder, achalasia etc.; some are of organic deformation, such as various kinds of organ volvulus, diverticulum and hernia; and some originated from outside gastrointestinal tract (e.g., pancreas, gall bladder, ovarium and uterus). It is doubtless that the first choice for physical diagnosis is to judge several aspects of these lesions mentioned above simultaneously. MPGIR which possesses the advantage of both single-contrast and double-contrast GIR, can not only examine organ morphologic changes caused by the diseases themselves or adjacent organs, but also identify organic functional changes. It can satisfactorily perform various examinations for the whole gastrointestinal tract, including multiphasic hypotonic upper gastrointestinal radiography, small bowel enema SBE, GI series, and double contrast enema DCE. That can meet the demand of clinical doctors as the first choice examination.
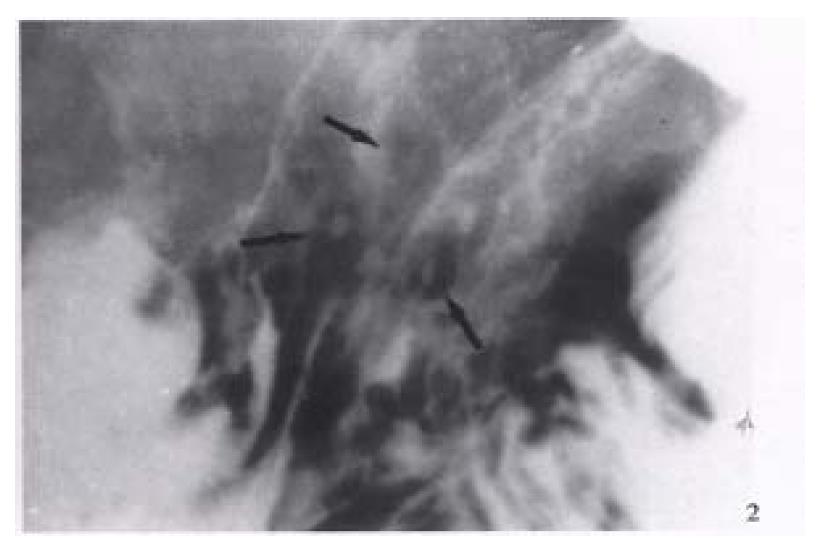
Figure 2 Erosive gastritis.
Multiple varioliform erosions (arrows) in the posterior wall of gastric body are seen as tiny barium collections with surrounding halos of edematous mucosa-“target” sign.
AN OVERALL DIAGNOSTIC MODALITY FOR GI TRACT
For the needs of planning the therapy and predicting prognosis correctly, four targets must be considered: site, sickness (qualitative determination), structure (quantitative determination), staging. That is the so called four “S” diagnosis. The basis of four “S” diagnosis is pathomorphologic and patho-physiologic changes[17]. All of the physical examinational approaches (endoscopy, radiography, ul-trasonography and endoscopic ultrasonography, MR, etc.) are all expected to reflect the pathologic changes objectively, and all kinds of examinational methods must be based on these criteria.
Refined MPGIR, which can obtain a complete view of the motion, the adoption, the ejection of organs and the pathophysiologic situation of the diseases, especially the location, shape, pathologic changes (different size, degree and extent) caused by mucosal or submucosal lesions, is superior to other techniques[18].
To detect the lesion-site
With full use of filling phase and double-contrast phase, MPGIR can easily obtain a complete view of the organ. Based on this, it can detect the abnormality of organ location, size and shape, making it easy to diagnose organ volvulus, diverticulum, hernia and so on[14]. According to the different organic changes caused by the lesions and the correlation between lesions and organs, MPGIR can clearly establish the origination (such as from the organ itself, the intramural or the extramural) of lesions for most of the patients. It can also show the pattern of lesions, whether protruding or depressing or both.
To determine the nature of the lesion-sickness
The nature of the lesion of gastrointestinal tract can be determined by its specific signs which appears in MPGIR under different contrast conditions (filling, mucosal, double-contrast, compressed phase) (Figure 3). Many these effective signs have been found by the radiologists, such as “bull eyes sign” indicating submucosal leiomyoma, “target sign” meaning erosion, “dimness, coarse, rigid sign” hinting malignant tumor, “step-like mucosal pattern” suggesting early gastric cancer, etc[8]. The better technical quality the more truthful im age and the more specific appearance the more accurate diagnosis[15,19,20].
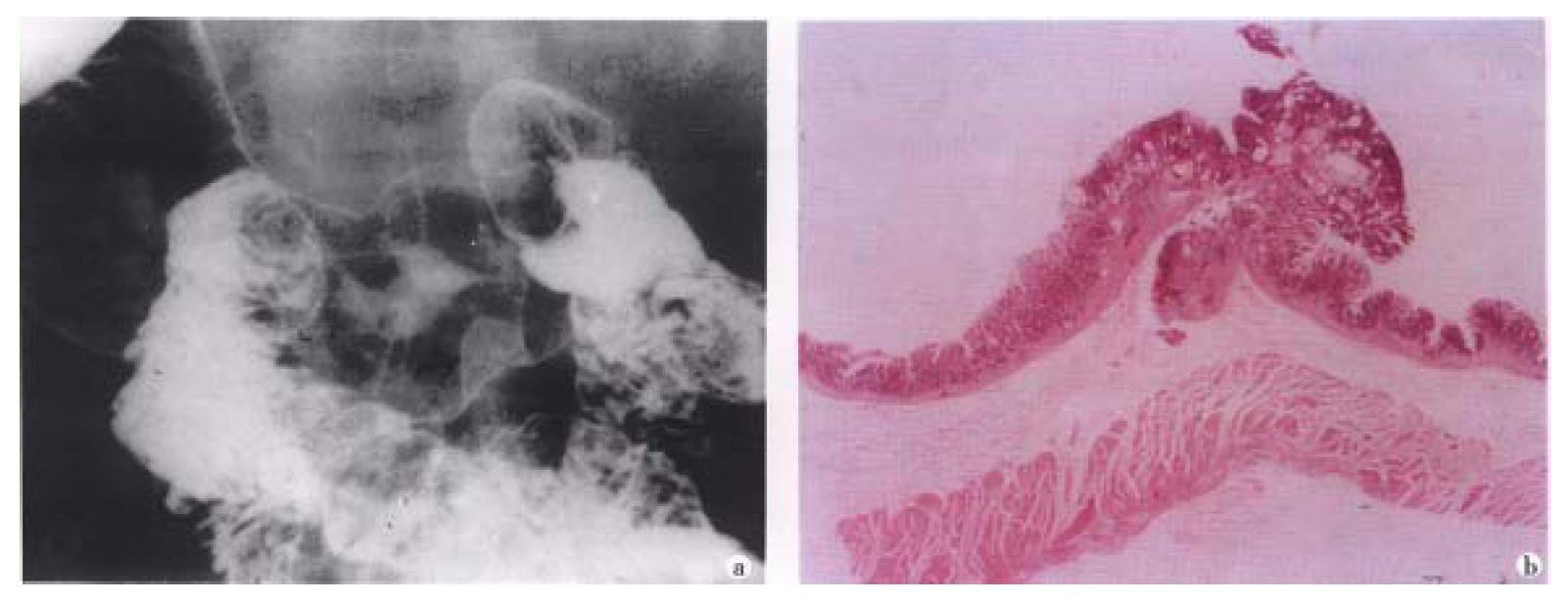
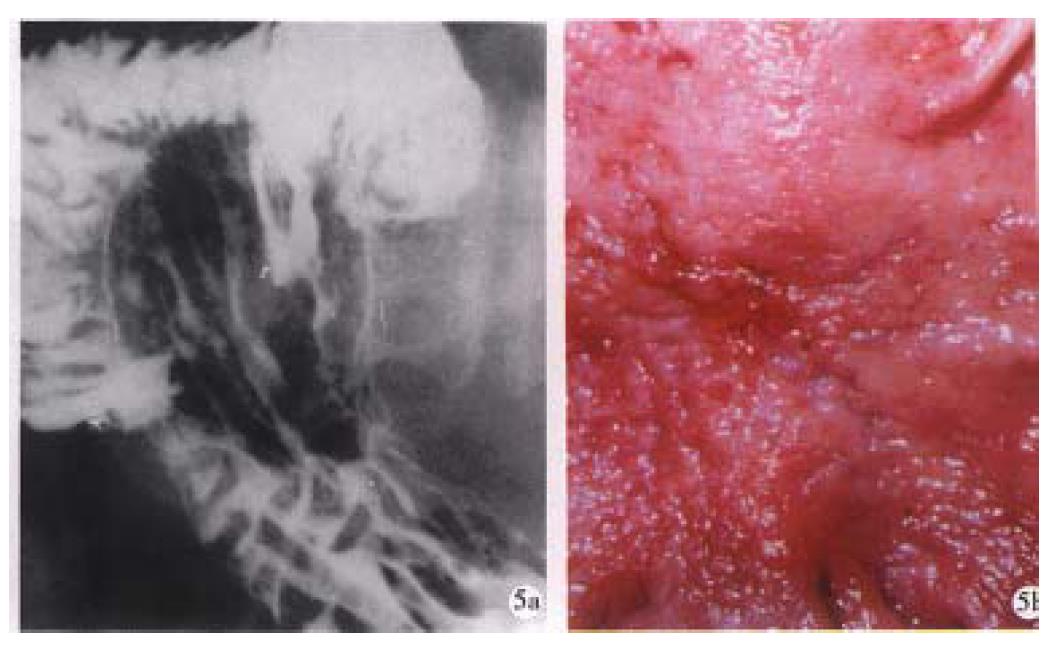
Figure 3 Small early gastric cancer (type I).
A sessile protruded lesion (5 mm in size) with its base puckered into the lumen is seen on the greater curvature of antrum.B. Micrograph shows the lesion originates from mucosa and infilt rates to the submucosal layer.
To identify the degree of the lesion-structure
Inflated by gas, the lesion outlined by barium is very clear and complete in MPGIR. It is very valuable for judging the size, extent and structure of lesions[14]. For example, in Borrmann’s type I cancer, it can clearly provide the overview of the size and the shape of the tumor protruded into the lumen, and can also obviously survey the gastric wall involved by the tumor, which is beneficial for surgery (Figure 4). Multiple primary carcinoma (MPC) of gastrointestinal tract is not rare, it can occur at either the same organ, such as multiple esophageal carcinomas, multiple gastric carcinomas or different organs, such as multiple esophagogastric carcinomas and multiple esophagogastrorectal carcinomas[21]. Preoperative discovery and diagnosis of all these lesions are especially important. It is less difficult to make diagnosis for MPGIR if more attention is paid to it. Nevertheless, other physical diagnostic approaches can hardly detect all the lesions, which might cause fatal results. For diagnosing gastro intestinal benign or functional disorders, the concept of “structure” still exists, such as evaluating the number and the size of ulcers, the type and the volume of reflux by using MPGIR.
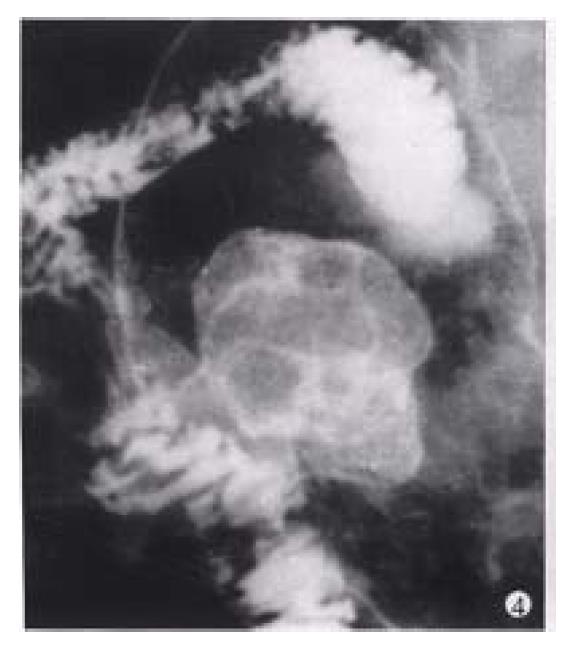
Figure 4 Gastric carcinoma (Borrmann’s type I).
A bulky cauliflower-like mass etched in white by a thin layer of barium is seen on the greater curvature of the stomach.
To ascertain the stage of the lesion-staging
MPGIR can sometimes ascertain the stage of the lesion in diagnosis. A niche suggests “active period” of the ulcer, a linear barium shadow means “healing period”, and a scar formation indicates “healed period” of the ulcer (Figure 5)[11]. To stage of carcinoma, the present signs can be used for initial judgement, for example, the special changes of mucosal folds imply early cancer, the “stiff gastric wall” sign suggests advanced cancer (Figure 6)[8]. However, the preoperational staging of cancers should be combined with CT examination[17,22].
Figure 5 Linear ulcer.
A long linear ulcer paralleled to the lesser curvature (arrowhead) shown on the gastric posterior wall, representing the healing and healed stage of ulcer. B. Macrograph shows that the fine curve linear ulcer is composed of re-epithelialized (healed) and granulating part (healing).
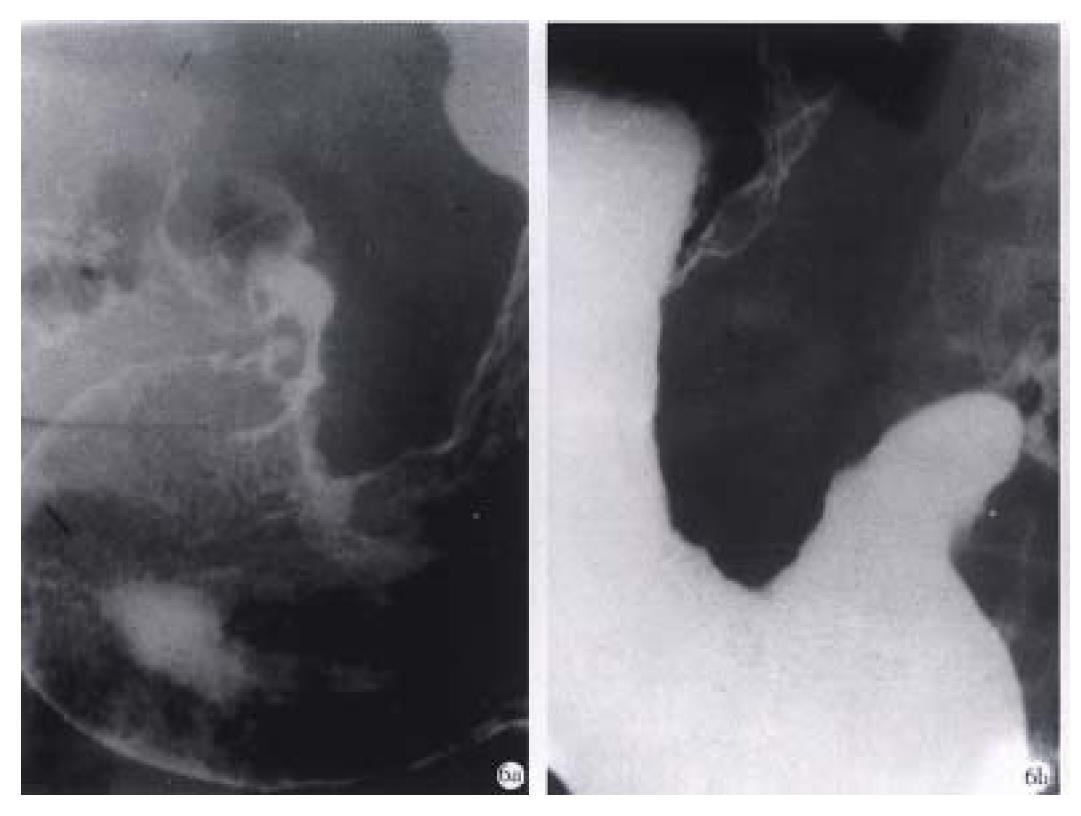
Figure 6 Advanced gastric carcinoma.
A. Double-contrast phase. B. Filling phase reveal marked “rigid” and “coarse” contour of the lesser curvature and lumen narrowing caused by infiltrative carcinoma.
THE INNOVATION OF GASTROINTESTINAL (GI) RADIOGRAPHY TECHNIQUE
The traditional GI radiography has experienced the following periods: local-con trolled fluoroscopic radiography, remote-controlled image intensifier, TV display, spot film[1], etc. In recent years, with the development of high-speed and high-efficiency computer, radiologic diagnosis has gradually become a new filmless technique using digital and postprocessing imaging systems. Digital GI imaging set on the basis of satisfactory double contrast GI technique possesses many advantages as follows which routine spot-film and fluororadiography do not have[23,24].
Rapid sequence ( 0.5-15 pictures/sec) images
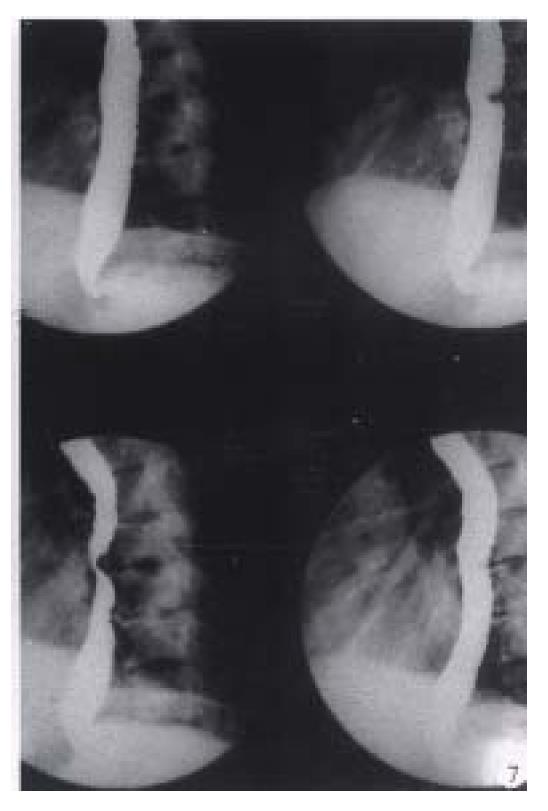
Rapid sequence images is very helpful for surveying the moving gastrointestinal tract, especially in the oral pharynx and the upper esophagus. As sufficient esophageal dilatation and mucosa coating can only keep on comparatively a short time, with the pulsation of heart, the high-quality D.C. films are not easy to acquire. Digital sequence exposure (0.5-2 pictures/sec) which can overcome these deficiencies, is obviously better than traditional imaging technique, for obtainning multiphasic images of esophagus (Figure 7)[25].
Figure 7 Digital esophageal barium radiography.
The defect on posterior aspect of middle esophagus caused by the proliferation of thoracic vertebrae is easily displayed by rapid sequence images (0.5 pictures/sec) and results in dysphagy clinically.
Quality control for the study
Every continuous picture can be accommodated on the monitor as a “frozen image”, until the next fluoroscopy begins. It can give the operator a clue whether the lesion is displayed satisfactorily[24]. If not, the doctor can adopt their apt procedure: changing projective position, mucosa recoating, and some more digital images in order to avoid the lesions missed due to slipshod examinational technique. Therefore, it can guarantee the quality of MPGIR, and increase the detectability of lesions.
Postproccessing
Digital imaging is not the same as the routing radiography, which can change the parameters in workstation, regulate the brightness and contrast of the picture, magnify the interesting area, adjust the edge enhancement, and annotate and print findings, and enter the picture archiving and communication system (PACS). All of these are making MPGIR enter into a brand new information era.
In summary a high-quality MPGIR can not only diagnose gastrointestinal diseases or disorders, but also make more complete judgement at the aspects of “site”, “sickness”, “structure” and “stage” provided by MPGIR[14,18,22]. It is charaterized by its own speciality and advantages: the convenient operation, and less sufferings caused to patients. So, it is still an important and the first choice examination. Both MPGIR and endoscopy must be adopted in a complementary manner to make up each other’s deficiencies, but not to be replaced one another. Of course, it is essential to maintain and improve the quality of MPGIR constantly. It is gratifying that the use of MPGIR examinations is increasing by 9.8% after 1996 in spite of its decline in the earlier 1990s according to the data from three big comprehensive hospitals in Shanghai. The current gastrointestinal radiology is one of the valuable medical treasures accumulated by many generations of scientists[8]. We should practise it continuously and explore, develop and improve it constantly, and do our best to contribute to gastrointestinal radiology.