Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.388
Revised: August 22, 1998
Accepted: September 26, 1998
Published online: October 15, 1998
AIM: To establish a tissue-specific gene therapy for colorectal carcinoma using bacterial ADP-ribosylating toxin genes.
METHODS: Pseudomonas exotoxin A domain II +III (PEA) was cloned from genomic DNA of Pseudomonas aeruginosa. PEA and diphtheria toxin A chain gene (DTA) were modified to express eukaryotically. After sequencing, the toxin genes under the control of human carcinoembryonic antigen (CEA) promoter were cloned into retroviral vectors to construct CEAPEA and CEADTA respectively. In vitro cotransfection of the constructs with luciferase vectors and in vivo gene transfer in nude mice were subsequently carried out.
RESULTS: Both CEAPEA and CEADTA specifically inhibited the reporter gene expression in the CEA positive human colorectal carcinoma (CRC) cells in vitro. Direct injection of CEAPEA and CEADTA constructs into the established human tumors in BALB/c nude mice led to significant and selective reductions in CRC tumor size as compared with that in control groups.
CONCLUSION: The toxin genes, working as therapeutic genes, are suitable for the tissue-specific gene therapy for colorectal carcinoma.
- Citation: Cao GW, Qi ZT, Pan X, Zhang XQ, Miao XH, Feng Y, Lu XH, Shigeki K, Ping D. Gene therapy for human colorectal carcinoma using human CEA promoter controled bacterial ADP-ribosylating toxin genes: PEA and DTA gene transfer. World J Gastroenterol 1998; 4(5): 388-391
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.388
A major requirement for in vivo gene therapy for cancer is to target the therapeutic gene expression to malignant tissues by selecting potent therapeutic genes. Pseudomonas exotoxin A and diphtheria toxin A chain, which kill tumor cells by irreversible ADP-ribosylation of elongation-2, may become potent therapeutic genes. PE is made of three structural domains: the N-terminal domain ( I ) is responsible for the binding of toxin to its receptor on the cells; the middle domain (II) plays a role in the translocation of toxin across the membrane, and the C-terminal domain (III) has the ADP-ribosylation activity[1]. DT is secreted from-Corynebacterium diphtheriae as a single polypeptide chain containing two major domains: A (amino-terminal, 193 residues), which carries the active site for ADP-ribosylation of elongation factor-2, and B (carboxyl-terminal, 342 residues), which promotes binding of toxin to cells and the entry of A into the cytosolic compartment. Immunotoxins have been engineered by fusing domains II and III of PE and DTA chain to specific antibodies. Immunotoxin therapy is challenged by limited accessibility of antibodies or antibody conjugates to solid tumors[2]. Expression of the toxin genes in mammalian cells may result in the inhibition of further protein biosynthesis, and consequent cell death.
In vivo gene therapy relies on unique tissue-specific or tumor-specific transcriptional elements to drive the expression of the toxic protein only in those cells containing transcription factors capable of activating the promoter elements. CEA is a tumor-associated cell surface antigen overexpressed in tumor cells such as colorectal carcinoma cells. CEA promoters were cloned both from normal and CEA-positive human colonic carcinoma genomic DNA[3]. Analysis of the CEA promoters showed more than 8-fold increase in expression in CEA-positive cells, as compared with CEA-negative cells[4]. We are working on an alternative means of exploiting a natural toxin which does not depend on cell surface molecules. Instead, DNA coding for the toxin genes driven by CEA promoter is introduced into tumor cells. The study may provide some opportunities for tissue-specific gene therapy for CEA-positive tumors.
CEA-positive LoVo and SW1463 human colorectal carcinoma cell line, HeLa cell line and murine embryo fibroblast NIH3T3 were originally obtained from ATCC. CEA-negative human renal cell carcinoma cell line RC9406 was established by our laboratory. BALB/c nude mice were from the Shanghai Tumor Research Institute ( Shanghai, China). All cells line and animals were maintained under standard conditions.
Pseudomonas aeruginosa strains were kept in our department. The sequence of oligonucleotide primers for cloning PEA domain II + III gene and DTA gene were obtained from published data[1,5], introducing Hind III site and kozak sequence at the 5’ terminus and strong termination signal and Cla I site at the 3’terminus, and commercially synthesized (Promega). Inner primers for cloning PEA gene had the sequence of GGAAGCTTGCCGCCACCATGGAGACTTTCACCCGTCATCGCCA and GGATCGATTTACTTCAGGTCCTCGCGCGGC. Outer primers for cloning PEA gene had the 5’sequence of ACGGTCATCAGTCATCGCCTGCA and shared the same 3’sequence with the inner primer. Oligonucleotide primers for cloning DTA had the sequences of GGAAGCTTGCCGCCACCATGGGCGCTGATGATGTTGTTGA and GGATCGATTTATCCGACACGATTTCCTGCACAG. Preparation of the bacterial genomic DNA and Nest-PCR employed to clone the PEA gene and plasmid pET15b-DT (kindly provided by Dr. RJ Collier[5]), were performed as previously described[3], with some modification. Genomic DNA (0.1 μg) and Elongase (0.5 U) (GIBCO) were used. The PCR products were digested with Cla I and Hind III, purified by the Wizard PCR product purification system (Promega), and then subcloned into pSP72 vectors to create pSP72-PEA and pSP72-DTA. Sequences of the cloned PEA and DTA fragments were analyzed using the double-stranded DNA sequencing system (Promega). The pSP72-PEA, pSP72-DTA, Sp6 and T7 primers (Promega) served as temples and sequencing primers respectively. Automatic DNA sequencing was commercially performed.
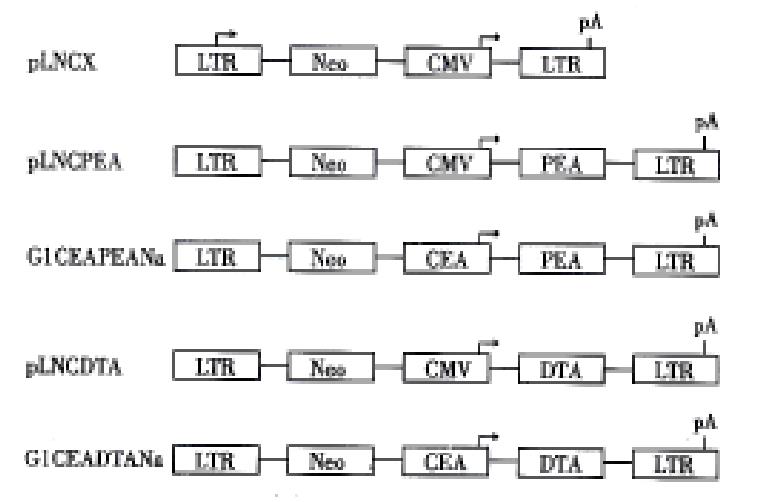
Sph I site of plasmid pGEM-CEA[3], which contained 400bp human CEA promoter cloned from genomic DNA of the CEA positive colorectal carcinoma tissues, was changed to Hind III. The Sal I/Hind III digested CEA promoter was released from pGEM-CEA, and subcloned into polylinker site of G1Na retroviral vector to create G1CEANa. The Hind III/Cla I digested PEA and DTA fragments were subcloned to G1CEANa and pLNCX[6] to create resulting constructs G1CEAPEANa, G1CEADTANa, pLNCDTA and pLNCPEA respectively. The structure of the constructs is shown in Figure 1.
Each transfection contained 1pmol pGL2 control (Promega) and 1pmol retroviral constructs. For transient luciferase assays, cells were transfected with DOTAP ( Boehringer Mannheim ). Transfection and luciferase assay were made as previously described[7]. All values were normalized to the expression of luciferase transfected with pGL2-control alone. All plasmids ( pLNCX, G1CEAP EANa, G1CEADTANa, pLNCPEA and pLNCDTA) were tested in SW1463, LoVo, NIH3T3, RC9406 and HeLa cell lines.
Two million LoVo and RC9406 cells were s. c. injected into the back of the BALB/c nude mice. When established, the tumors were resected and sheared to 0 mm-5 mm3, and s.c. reimplanted into the BALB/c nude mice of next generation under sterile conditions. After 30 generations of reimplantation, the tumors grew steadily in the nude mice.
After purification with column chromatography, the constructs (20 mg/L) were mixed with the same volume of lactated Ringer’s solution. An aliquot of the mixture was added to the same volume of a cationic liposome (composed of TMAG, DLPC and DOPE, with a molar ratio of 1: 2 :3) in lactated Ringer’s solution in a separate sterile vial at room temperature. After incubation for 15 min, 0.1 mL DNA liposome solution was injected into the tumors, or in the presence of 107 units per mL adenovirus ( Ad2 ), under sterile conditions. The injection was administered 6 times at an interval of 2 days. The tumor weight was estimated as previously described[8]. The mice were killed at 42 h following the last injection of the constructs, and then the treated tumors were resected. Total RNA was extracted with TriZol (GIBCO), and subsequently digested with RNAse-free DNAse I (GIBCO). RT-PCR for detection of the transcription of PEA and DTA gene were performed with the inner pairs of the related primers to confirm the toxin gene expression.
The Student’s t test and χ2 test were used. A P value of < 0.05 was considered significant.
The modified PEA and DTA gene were obtained. DNA sequencing demonstrated that the introduced Kozak sequence, termination code and restriction sites did not destroy the open reading frame of PEA and DTA coding regions. The PEA gene had no mutation in the aminoacid sites 485-492, 553 and 612, which were considered to be key positions for expressing ADP-ribosylation activity. Base-replacement mutation was found in the aminoacid positions 412 and 420. Roles of these mutations remained to be investigated. No mutation was found in the DTA gene.
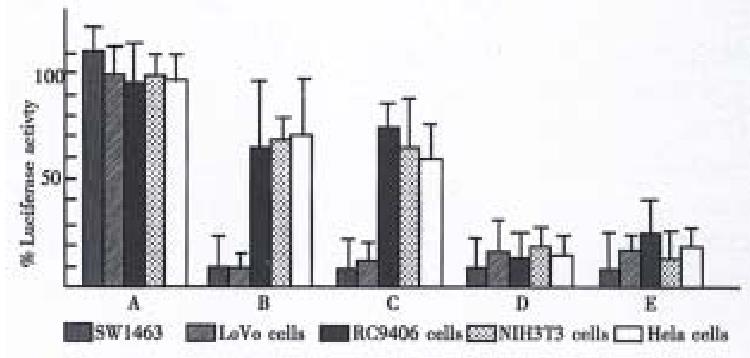
In vitro experiments showed that the CEA promoter conferred a selective expression of PEA and DTA gene in CEA positive human colorectal carcinoma cell lines LoVo and SW1463. The levels of luciferase expression in LoVo and SW1463 cells were inhibited by 90%-97% and 80%-85% at 36 h after transfection with an equal mass of G1CEAPEANa and G1CEADTANa respectively. Concentration dependent inhibition was also observed, the inhibition being substantial with as little as 0.1 μg. In contrast, cotransfection with a plasmid lacking the PEA and DTA genes did not inhibit luciferase expression (Figure 2). The basal transcription level of the PEA and DTA from the CEA promoter was inferred from a partial translation inhibition effect of the cotransfected luciferase gene in the CEA negative RC9406 and HeLa cells. The results indicate that the translation inhibition of luciferase was due to active toxic protein production and level of the inhibition was closely associated with PEA and DTA gene expression.
The growth rate of LoVo tumor in the groups treatment with G1CEAPEANa and G1CEADTANa appeared to be reduced as compared with the liposome controls at day 7 of the treatment. Growth of RC9406 tumors did not significantly respond to G1CEAPEANa or G1CEADTANa administration (P > 0.05, compared to the liposome control). The selective anti-colorectal carcinoma effect of G1CEAPEANa was stronger than G1CEADTANa (P < 0.05, n = 7). pLNCPEA and pLNCDTA i.t. injections with the cationic liposome were relatively capable of reducing the growth rate of both LoVo tumors and RC9406 tumors (P < 0.05, as against liposome control). Intratumoral administration of G1CEAPEANa in the presence of the adenovirus (Ad2 type) and cationic liposome resulted in marked regression of the established colorectal carcinoma, as compared with G1CEAPEANa and the liposome groups. The ADP-ribosylating bacterial toxin genes, under the control of the CEA promoter, showed strong and selective antitumor activities for CEA positive CRC tumor (Table 1). RT-PCR employed for the detection of the toxin gene expression in the treated tumor bodies showed transcription of the toxin genes in G1CEAPEANa treated or G1CEADTANa treated LoVo tumors and pLNCPEA treated or pLNCDTA treated LoVo and RC9406 tumor, but not in G1CEAPEANa or G1CEADTANa treated RC9406 tumor bodies. The results demonstrated that expression of the toxin genes was responsible for the growth inhibition of the tumor bodies.
| Days following gene therapy | Estimated tumor weight (mg) of tumor xenograft following toxin genes transfer (-x±s, n = 7) | ||||||||
| LoVo (CEA/PEA) | RC9406 (CEA/PEA) | LoVo (CEA/DTA) | RC9406 (CEA/DTA) | LoVo (CMV/PEA) | RC9406 (CMV/DTA) | LoVo | RC9406 | LoVo (CEA/PEA) + Ad | |
| 0 | 486 ± 160 | 472 ± 201 | 504 ± 232 | 516 ± 164 | 480 ± 200 | 496 ± 320 | 480 ± 190 | 490 ± 230 | 490 ± 130 |
| 7 | 404 ± 176 | 632 ± 246 | 480 ± 320 | 706 ± 240 | 365 ± 240 | 380 ± 224 | 680 ± 185 | 680 ± 210 | 414 ± 98 |
| 21 | 454 ± 201 | 1246 ± 212 | 645 ± 340 | 1321 ± 324 | 584 ± 402 | 638 ± 320 | 1386 ± 440 | 1206 ± 340 | 226 ± 63 |
| 35 | 758 ± 424 | 1840 ± 764 | 1084 ± 286 | 1986 ± 884 | 906 ± 327 | 986 ± 380 | 2810 ± 528 | 2762 ± 620 | 306 ± 45 |
| (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 5) | |||||
Both DTA and PEA coding genes can be modified to express prokaryotically and eukaryotically, and post-translational process in eukaryotic cells did not eliminate its ADP-ribosylation activity. So the toxin genes are suitable for cancer gene therapy if expression of the genes can be restricted into tumor cells or tumor bodies. In the study, cell type-specific inhibition and concentration-dependent inhibition of luciferase gene expression were demonstrated in the cotransfection assay, implying that expression level of the toxin genes played a crucial role in the growth inhibition of the established human tumor. Our previous studies suggested that tissue and tumor-specific transcriptional regulatory sequences played a central role in conferring a selective expression of prodrug activation gene[8] and cytokine genes[9-11]. We cloned CEA promoters and found that some trans-acting elements residing in the nuclei of the colorectal carcinoma cells LoVo, not in RC9406 renal carcinoma cells, proved to be responsible for the CEA overexpression in the cancer cells, and the flanking region of CEA gene lacked the conventional TATA and CAAT boxes. The results of these studies indicated that the CEA promoter might be strict enough to confer the toxin genes to selectively express in CEA positive colorectal carcinoma. From in vitro studies, the specific expression of the toxin genes driven by the CEA promoter was demonstrated following cotransfection with the luciferase vectors, but the basal transcription of PEA and DTA gene in CEA negative cells may exist. Tissue or cell specificity of transcriptional regulatory elements for controlling expression of these genes remains to be improved. The prokaryotic transcriptional control systems, the tetracycline system and the lactose system, have been employed to control expression of DTA gene in glioma gene therapy[12]. A more practicable control system needs to be developed.
In the present study, the retroviral constructs were respectively introduced into ecotropic and amphotropic retrovirus packaging cells in order to generate recombinant retroviruses. But we failed to obtain a stable retrovirus producing cell line. Concentration of the retrovirus was too low to be measurable, although HIV-regulated DTA transcription unit was reported to have been packaged into recombinant retroviruses. Intratumoral injection of the unique retroviral constructs with a cationic liposome was the only alternative way for the in vivo gene therapy. The liposomal delivery system for the toxic gene is suitable for the in vivo gene therapy for preparation of liposome using cationic lipids has also become extremely popular for the delivery of pDNA, largely due to its simplicity and relative effectiveness. Cationic liposome-mediated expression of viral promoter regulated DTA gene has been used for the treatment of HIV infection[13]. In the study, the liposome delivered PEA or DTA genes under the control of the CEA promoter resulted in selective inhibition on the growth of CEA positive colorectal carcinoma in the animal models. The inhibition would be stronger if the Ad2 adenoviruses were added into the gene transfer system. The addition of adenoviruses in trans together with the conjugates has resulted in a significant increase in the transfection activity, probably because of its ability to aid the escape of the conjugate from the endosomal-lysosomal pathway[14]. The strategy may be useful in clinical investigation.
Presented at the Annual Meeting of American Association for Cancer Research, Philadelphia, 24-28 March, 1998.
| 1. | Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 253] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Chen SY, Yang AG, Chen JD, Kute T, King CR, Collier J, Cong Y, Yao C, Huang XF. Potent antitumour activity of a new class of tumour-specific killer cells. Nature. 1997;385:78-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Cao G, Kuriyama S, Gao J, Mitoro A, Cui L, Nakatani T, Zhang X, Kikukawa M, Pan X, Fukui H. Comparison of carcinoembryonic antigen promoter regions isolated from human colorectal carcinoma and normal adjacent mucosa to induce strong tumor-selective gene expression. Int J Cancer. 1998;78:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Cao GW, Du P, Pan X, Zhang XQ, Cui L, Qi ZT. Identifying the role of CEA core promoter region from human colorectal carcinoma tissues in the cancer-specific cytosine deami nase gene therapy. WJG. 1998;in press. [Cited in This Article: ] |
| 5. | Silverman JA, Mindell JA, Finkelstein A, Shen WH, Collier RJ. Mutational analysis of the helical hairpin region of diphtheria toxin transmembrane domain. J Biol Chem. 1994;269:22524-22532. [PubMed] [Cited in This Article: ] |
| 6. | Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 321] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Richards CA, Austin EA, Huber BE. Transcriptional regulatory sequences of carcinoembryonic antigen: identification and use with cytosine deaminase for tumor-specific gene therapy. Hum Gene Ther. 1995;6:881-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Cao GW, Qi ZT, Pan X, Zhang XQ, Gao J, Cui L et al. Gene therapy for malignant melanoma using bacterial cytosine deaminase gene under the control of tyrosinase promoter. J Med Coll PLA. 1998;13:in press. [Cited in This Article: ] |
| 9. | Cao G, Kuriyama S, Du P, Sakamoto T, Yang W, Masui K, Qi Z. Construction of retroviral vectors to induce strong hepatoma cell-specific expression of cytokine genes. J Gastroenterol Hepatol. 1996;11:1053-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Cao G, Kuriyama S, Du P, Sakamoto T, Kong X, Masui K, Qi Z. Complete regression of established murine hepatocellular carcinoma by in vivo tumor necrosis factor alpha gene transfer. Gastroenterology. 1997;112:501-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Cao GW, Gao J, Du P, Qi ZT, Kong XT. Construction of retroviral vectors to induce a strong expression of human class I interferon genes in human hepatocellular carci-noma cells in vitro. Chin Natl J New Gastroenterol. 1997;3:139-142. [Cited in This Article: ] |
| 12. | Paulus W, Baur I, Oberer DM, Breakefield XO, Reeves SA. Regulated expression of the diphtheria toxin A gene in human glioma cells using prokaryotic transcriptional control elements. J Neurosurg. 1997;87:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Konopka K, Harrison GS, Felgner PL, Düzgüneş N. Cationic liposome-mediated expression of HIV-regulated luciferase and diphtheria toxin a genes in HeLa cells infected with or expressing HIV. Biochim Biophys Acta. 1997;1356:185-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Seth P, Brinkmann U, Schwartz GN, Katayose D, Gress R, Pastan Ira K. Adenovirus-mediated gene transfer to human breast tumor cells: an approach for cancer gene therapy and bone marrow purging. Cancer Res. 1996;56:1346-1351. [PubMed] [Cited in This Article: ] |