Copyright
©The Author(s) 2025.
World J Gastroenterol. Apr 14, 2025; 31(14): 104975
Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.104975
Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.104975
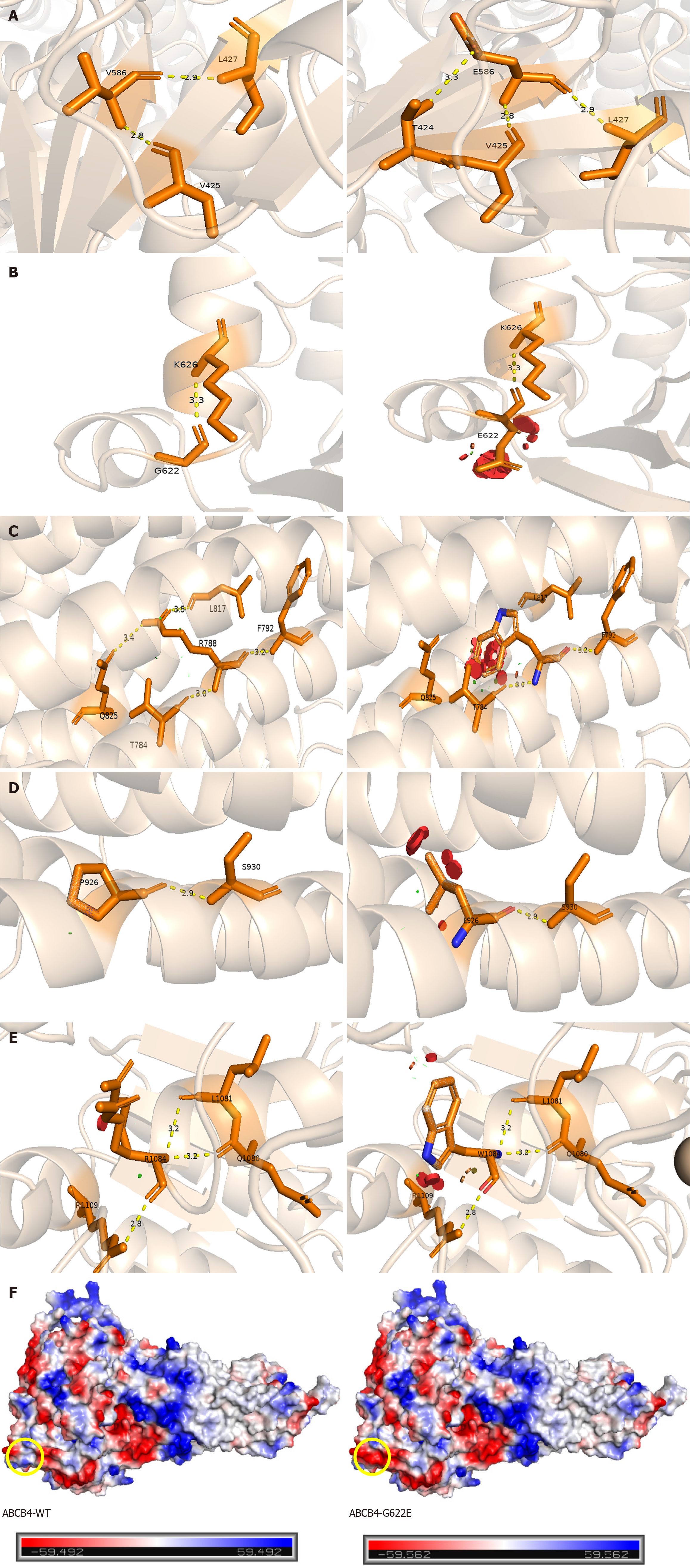
Figure 2 Structural analysis of missense variants in multidrug resistance protein 3.
Prediction of three-dimensional structure of ATP-binding cassette subfamily B member 4 (ABCB4) mutation sites by Swiss Model. A: P.V586E mutation forms new hydrogen bond with T424; B: P.G622E mutation affects steric hindrance; C: P.R788W mutation breaks hydrogen bond with Q825 and L817; D: P.P926 L mutation affects steric hindrance; E: P.R1084W mutation affects steric hindrance; F: The left image represents ABCB4-WT, while the right image illustrates the G622E variant. The yellow circle indicates that the neutral glycine is replaced by the negatively charged glutamic acid, with the red region representing negative charges, the white region indicating neutrality, and the blue region denoting positive charges. ABCB4: ATP-binding cassette subfamily B member 4.
- Citation: Weng YH, Zheng YF, Yin DD, Xiong QF, Li JL, Li SX, Chen W, Yang YF. Clinical, genetic and functional perspectives on ATP-binding cassette subfamily B member 4 variants in five cholestasis adults. World J Gastroenterol 2025; 31(14): 104975
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/104975.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.104975