Copyright
©The Author(s) 2024.
World J Gastroenterol. Dec 14, 2024; 30(46): 4904-4913
Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4904
Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4904
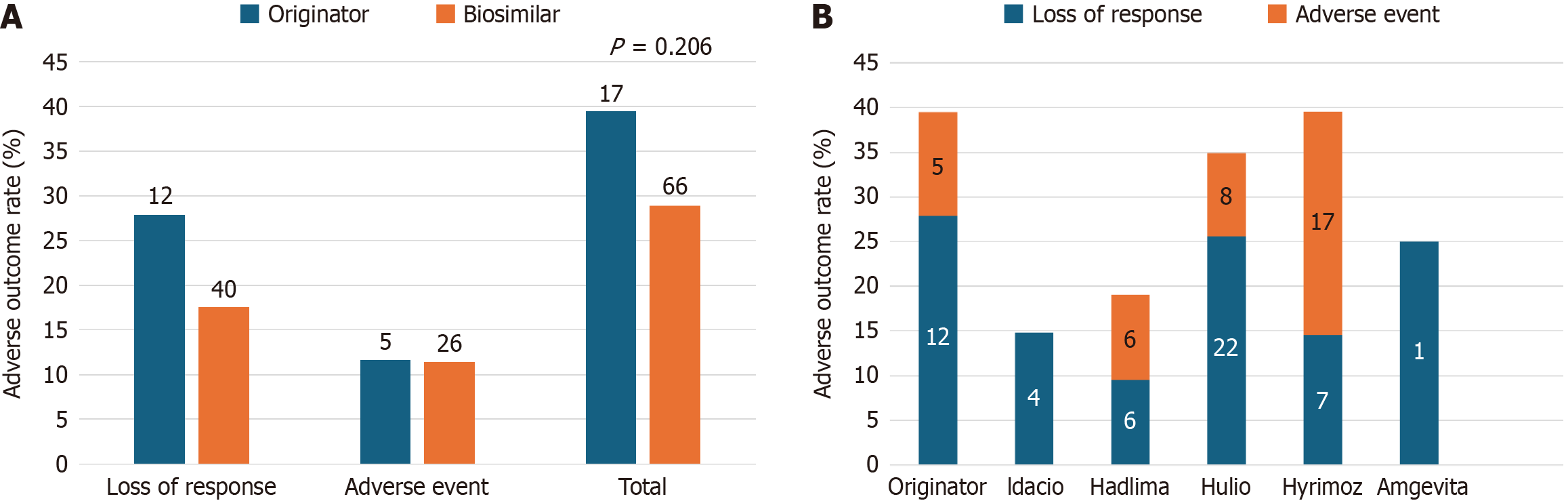
Figure 3 Adverse outcomes.
A: No statistically significant differences were observed in rates of adverse outcomes (regardless of subsequent treatment discontinuations) were observed between originator and biosimilar cohorts (P = 0.206); B: Rates of adverse outcomes subdivided amongst each biosimilar compared to the originator control group. N-values are indicated within bars.
- Citation: Liu Chen Kiow J, Hoang T, Bedi HK, Majdzadeh Ardekani Z, Rosenfeld D, Reise-Filteau M, Bressler B, Leung Y, Rosenfeld G. Real-world experience and long-term outcomes of a mandatory non-medical switch of adalimumab originator to biosimilars in inflammatory bowel disease. World J Gastroenterol 2024; 30(46): 4904-4913
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4904