Copyright
©The Author(s) 2024.
World J Gastroenterol. Nov 21, 2024; 30(43): 4620-4635
Published online Nov 21, 2024. doi: 10.3748/wjg.v30.i43.4620
Published online Nov 21, 2024. doi: 10.3748/wjg.v30.i43.4620
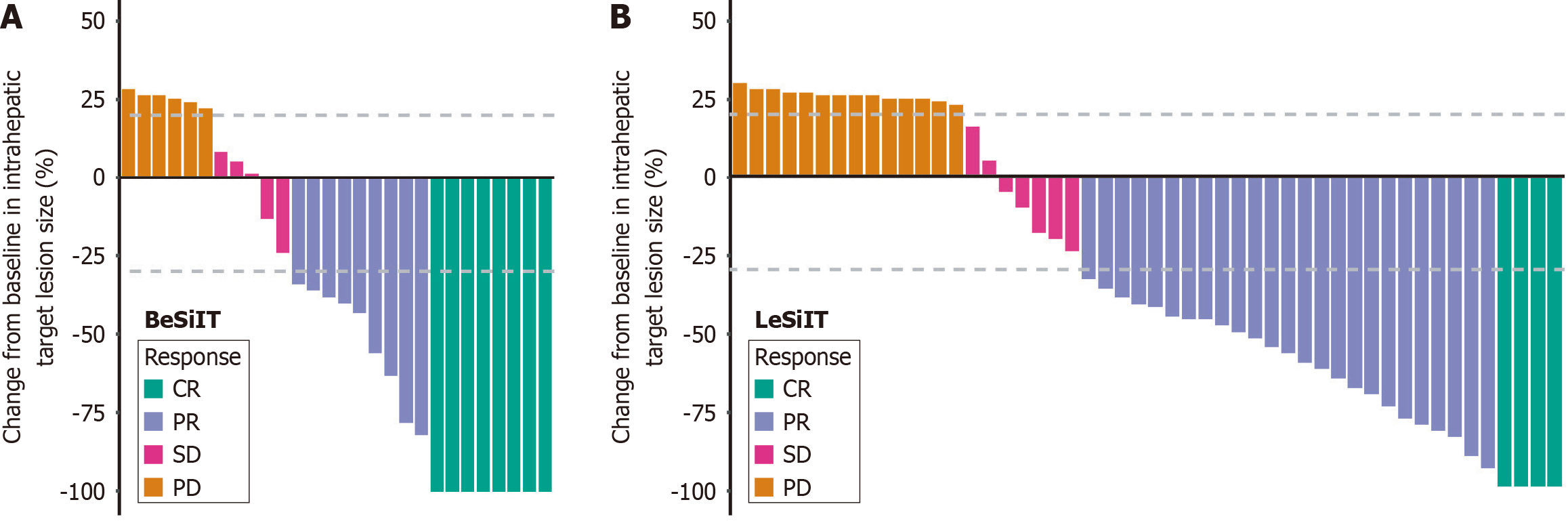
Figure 4 Best percentage changes in the sizes of the intrahepatic target lesions of patients from baseline assessed with modified response evaluation criteria in solid tumors.
A: Bevacizumab plus sintilimab plus interventional treatment; B: Lenvatinib plus sintilimab plus interventional treatment. The horizontal coordinate represents each patient and the vertical coordinate represents the percentage change in intrahepatic target lesion size from baseline. BeSiIT: Bevacizumab plus sintilimab plus interventional treatment; LeSiIT: Lenvatinib plus sintilimab plus interventional treatment; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
- Citation: Han RY, Gan LJ, Lang MR, Ren SH, Liu DM, Li GT, Liu YY, Tian XD, Zhu KW, Sun LY, Chen L, Song TQ. Lenvatinib, sintilimab combined interventional treatment vs bevacizumab, sintilimab combined interventional treatment for intermediate-advanced unresectable hepatocellular carcinoma. World J Gastroenterol 2024; 30(43): 4620-4635
- URL: https://www.wjgnet.com/1007-9327/full/v30/i43/4620.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i43.4620