Copyright
©The Author(s) 2024.
World J Gastroenterol. Jul 28, 2024; 30(28): 3428-3446
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
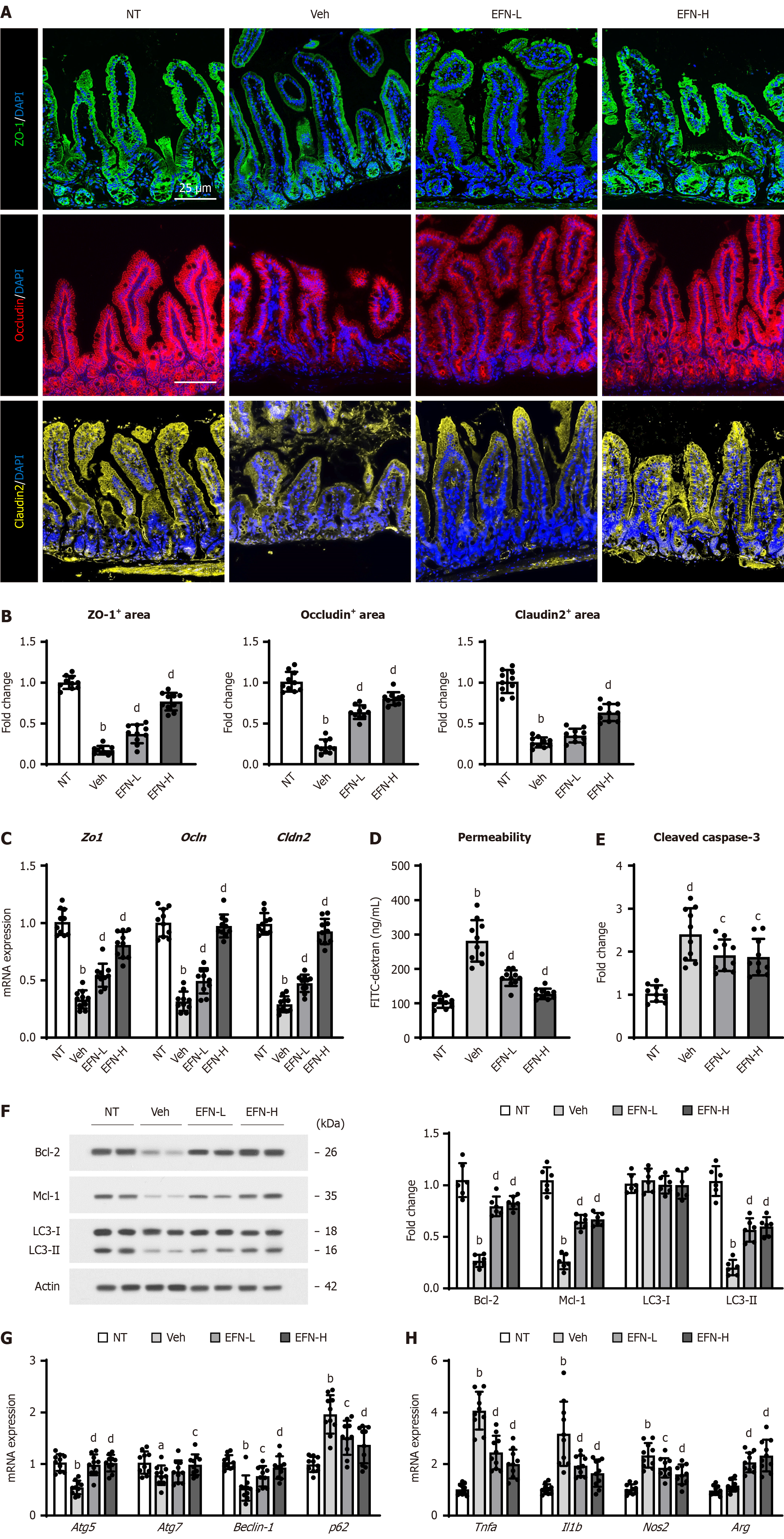
Figure 6 Elafibranor on intestinal barrier function in the alcohol-associated liver disease mice.
A: Representative microphotographs of ileum sections immunofluorescent stained with tight junction proteins (TJPs) including zonula occludens-1 (ZO-1), occludin and claudin-2; B: Quantitation of ZO-1, occludin and claudin-2 immunopositive area in high-power field (n = 10); C: Intestinal mRNA levels of TJPs (n = 10); D: Blood levels of fluorescein isothiocyanate-dextran (4 kDa) 4 hours after oral administration (n = 3); E: Cleaved caspase-3 level in the ileum tissue (n = 10); F: Western blot for the protein expression of Bcl-2, Mcl-1 and LC3-1 and 2 in the ileum tissue. Actin was used as an internal control; G and H: Intestinal mRNA level of the markers related to autophagy (G) and macrophage activation (H) (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (C, G, and H). Quantitative values are indicated as fold changes to the values of non-therapeutic group (B, C, E-H). Data are the mean ± SD. aP < 0.05 vs non-therapeutic group; bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; ZO-1: Zonula occludens-1; TNF: Tumor necrosis factor; IL: Interleukin; FITC: Fluorescein isothiocyanate.
- Citation: Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol 2024; 30(28): 3428-3446
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3428