Copyright
©The Author(s) 2024.
World J Gastroenterol. Jul 28, 2024; 30(28): 3428-3446
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
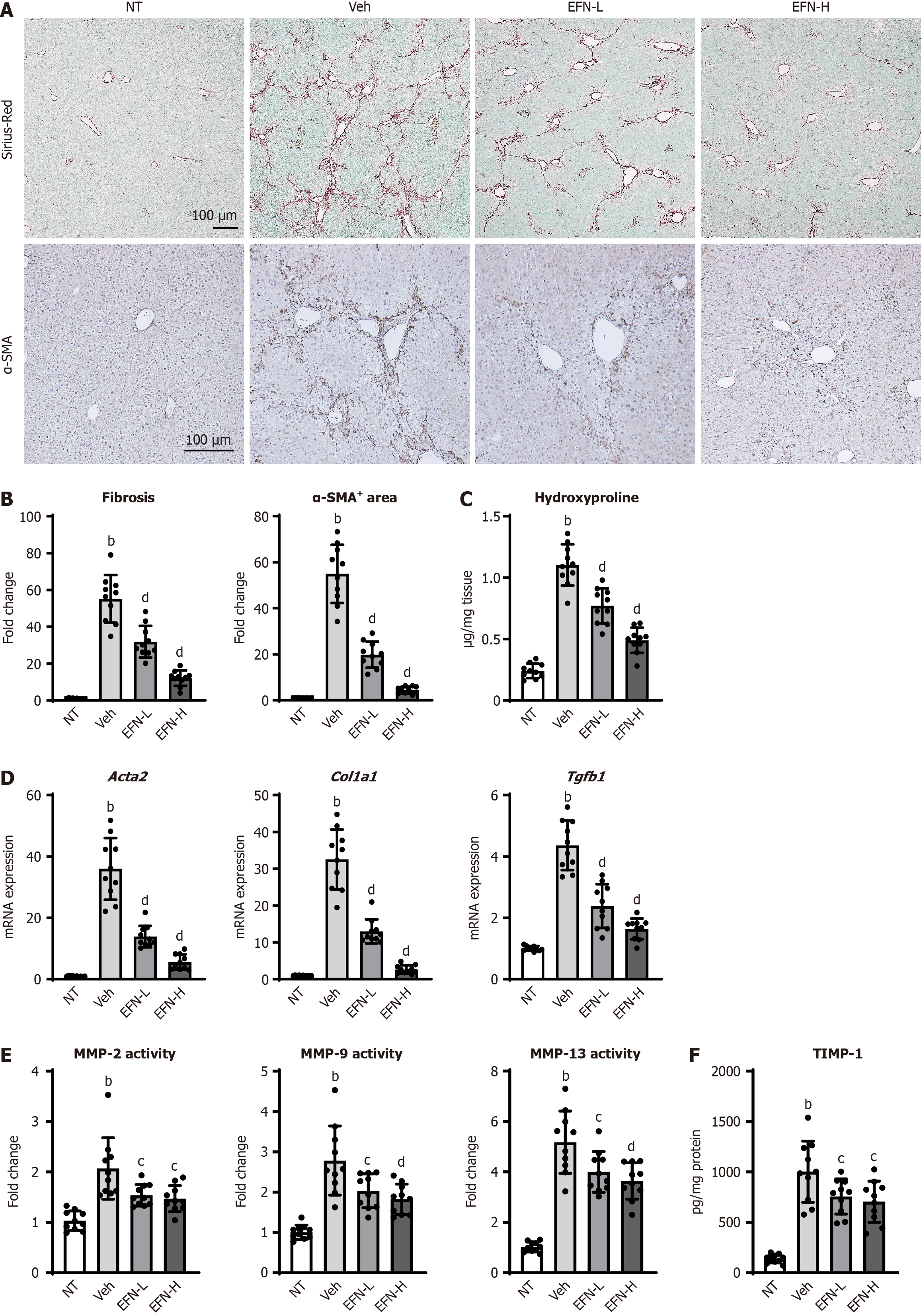
Figure 3 Elafibranor on hepatic fibrosis development in the alcohol-associated liver disease mice.
A: Representative microphotographs of sirius-red and α-smooth muscle actin (SMA) staining of the livers in the experimental mice; B: Quantification of sirius-red stained fibrotic area and α-SMA-positive area in high-power field (n = 10); C: Hepatic concentration of hydroxyproline (n = 10); D: Hepatic mRNA level of profibrotic markers (n = 10); E: Hepatic activity of matrix metalloproteinases (MMP)-2, MMP-9, and MMP-13 (n = 10); F: Hepatic level of tissue inhibitor of metalloproteinase 1 (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (D). Quantitative values are indicated as fold changes to the values of non-therapeutic group (B, D and E). Data are the mean ± SD. bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; α-SMA: α-smooth muscle actin; MMP: Matrix metalloproteinases; TIMP1: Tissue inhibitor of metalloproteinase 1.
- Citation: Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol 2024; 30(28): 3428-3446
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3428