Copyright
©The Author(s) 2024.
World J Gastroenterol. May 21, 2024; 30(19): 2553-2563
Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2553
Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2553
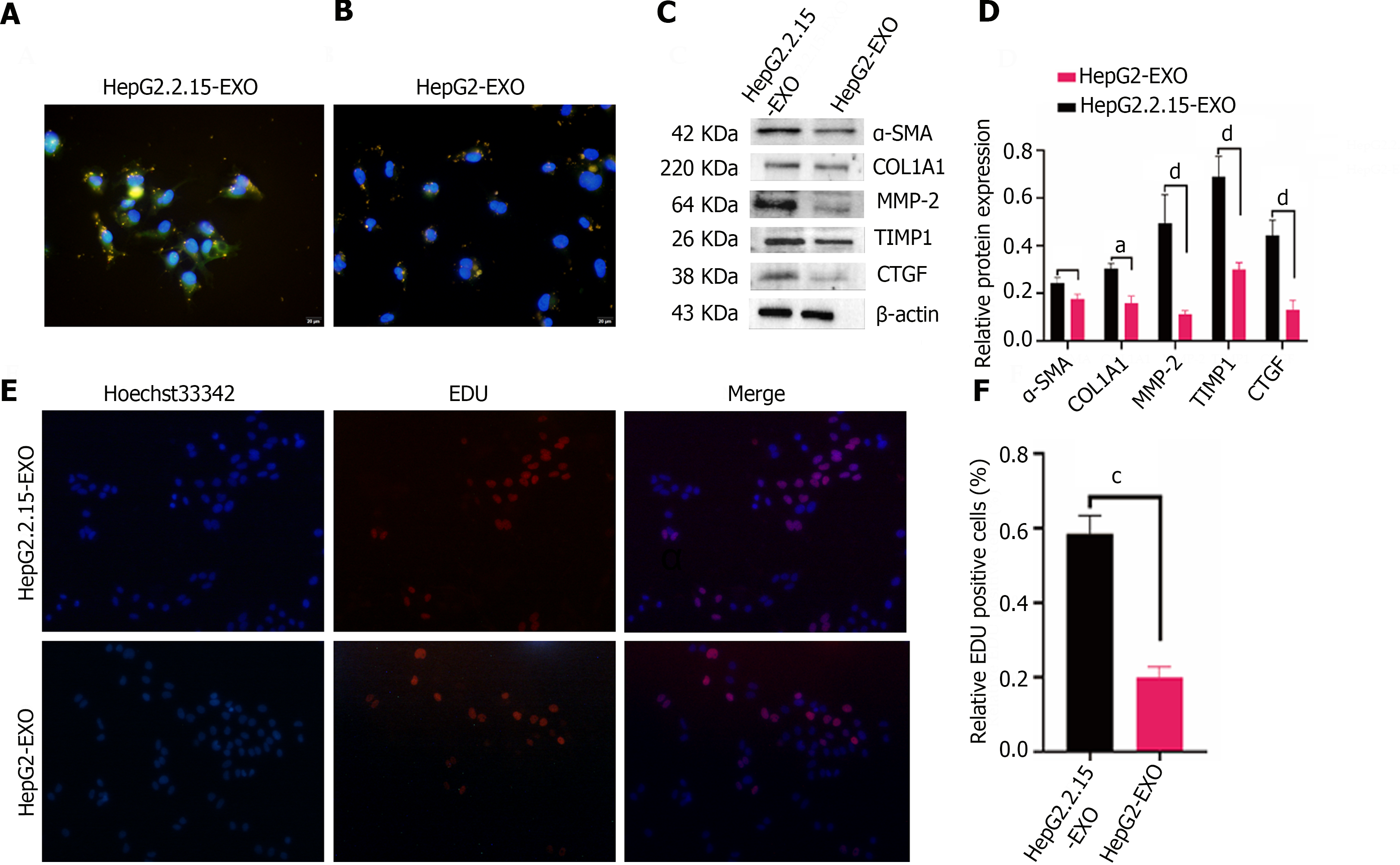
Figure 3 The role of HepG2.
2.15-derived exosomes in LX2 proliferation and fibrosis. A: HepG2.2.15-derived exosomes (colored in yellow), highly expressing α-smooth muscle actin (SMA) (shown in green), are illustrated as being internalized by LX2 cells. The cells showcase a spindled shape and notable pseudopodia with some exosomes penetrating the cell nucleus (depicted in blue). The scale bar signifies 100 μm for reference; B: The HepG2-derived exosomes (yellow) exhibit lower expression levels of α-SMA (green) within the cytoplasm of the internalizing LX2 cells. Again, several exosomes can be observed within the cell nuclei (shown in blue). The same scale is applied; C: The expression of fibrosis marker proteins, namely α-SMA, COL1A1 type, MMP2, TIMP-1, and CTGF, within LX2 cells are verified through a Western blotting assay; D: Quantitative analysis of Western blotting was done on the intensity of fibrosis marker protein bands; E and F: The 5-ethynyl-2′-deoxyuracil cell proliferation experiment presents that the exosomes secreted by hepatitis B virus-infected hepatocytes have the capabilities of promoting LX2 cell proliferation. A 100 μm scale bar is used (Error bars represent the mean ± SD; aP < 0.05; cP < 0.001; dP < 0.0001). EDU: 5-ethynyl-2′-deoxyuracil; α-SMA: α-smooth muscle actin.
- Citation: Gao Y, Li L, Zhang SN, Mang YY, Zhang XB, Feng SM. HepG2.2.15-derived exosomes facilitate the activation and fibrosis of hepatic stellate cells. World J Gastroenterol 2024; 30(19): 2553-2563
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2553.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2553