Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1655
Peer-review started: December 25, 2023
First decision: January 4, 2024
Revised: January 10, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: March 28, 2024
Processing time: 93 Days and 22.4 Hours
The gut microbiota is recognized as an endocrine organ with the capacity to influence distant organs and associated biological pathways. Recent advan
Core Tip: Maintaining intestinal microbial homeostasis is essential for human health. Dysbiosis of gut microbiota has been demonstrated in patients with polycystic ovarian syndrome, endometriosis, breast cancer, cervical cancer, and ovarian cancer, disordered gut microbiota may affect the occurrence and development of these diseases through the immune system, estrogen, or metabolite pathways. In the future, maintaining gut microbiota homeostasis may be a promising treatment.
- Citation: Wang MY, Sang LX, Sun SY. Gut microbiota and female health. World J Gastroenterol 2024; 30(12): 1655-1662
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1655.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1655
The human body contains trillions of microbes, rapidly diversifying after birth. Recent developments in genome sequencing, transcriptome analysis, and metabolomics have enabled researchers to explore the microbiota in more detail, particularly their functions. The gut microbiota plays a pivotal role in nutrient transformation and absorption, maintaining vital interactions with multiple tissues and organs, an indispensable factor for human health. The primary bacteria found in the gut are Firmicutes and Bacteroidetes, accounting for 90% of the flora in the gut[1], other bacterial constituents include Actinobacteria and Proteobacteria.
While a universally healthy gut microbiota remains undefined, dysbiosis has been associated with diseases ranging from irritable bowel syndrome to cancer. Previous studies have reported sex differences in the distribution of gut microbiota and disease prevalence[2], of which, the female gut microbiota emerges as a compelling area for investigation. Marano et al[3] underscore the strategic role of gut microbiota in crucial life stages for women, from childhood through adolescence, fertile age to pregnancy-partum, and up to menopause. This editorial aimed to discuss the potential mechanisms and therapeutic targets associated with the impact of gut microbiota dysbiosis on female diseases.
The gut microbiota can both promote and inhibit inflammatory response by influencing inflammatory factors, thus affecting the onset and progression of female diseases. In a study by Xu et al[4], ovarian cancer cells transplanted into mice with gut microbiota dysbiosis demonstrated increased xenograft tumor sizes. This dysbiosis stimulated macrophages, resulting in increased circulating levels of interleukin (IL)-6 and tumor necrosis factor-α, thus inducing the progression of ovarian cancer epithelial-mesenchymal transition[4]. One study examined the gut microbiota in patients with preeclampsia, revealing that Akkermansia muciniphila significantly suppressed inflammation and alleviated preeclamptic symptoms in rats by promoting autophagy and M2 polarization of macrophages in the placental bed[5]. Dysbiotic shifts in the gut microbiota are associated with increased gut permeability, leading to increased translocation of bacterial endotoxins, primarily lipopolysaccharide (LPS)[6]. The activation of the innate immune system through toll-like receptor 4 by LPS increases the expression of proinflammatory cytokines via nuclear factor κB translocation[7].
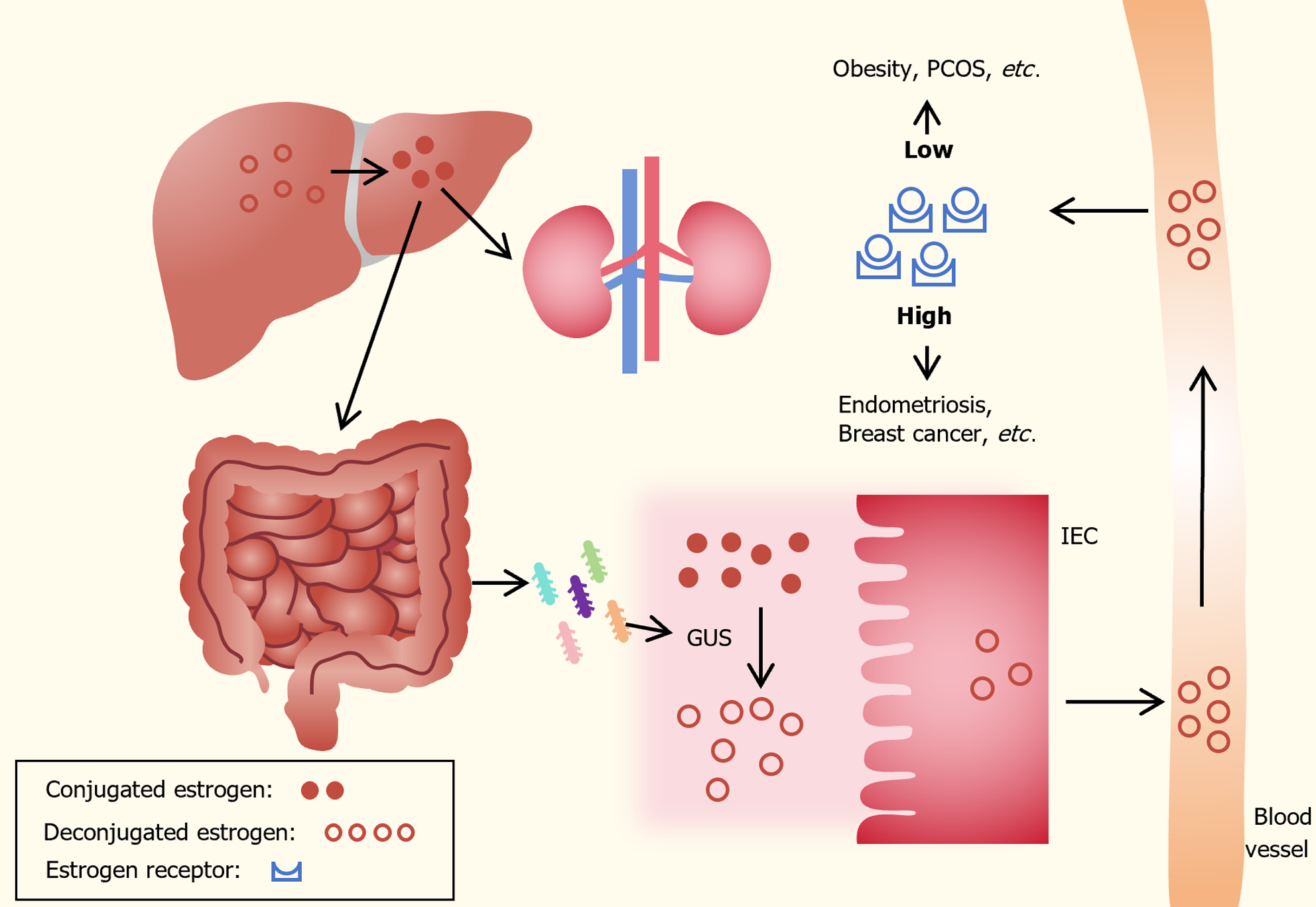
In premenopausal women, ovaries use cholesterol derived from saturated fats for estrogen synthesis. Following menopause, adipose tissue, adrenal glands, and other organs convert circulating androgens into estrogens through the aromatase enzyme[8,9]. As shown in Figure 1, circulating estrogens undergo conjugation in the liver through glu-curonidation or sulfonation, facilitating their excretion in bile, urine, and stool. The gut microbiota significantly influences estrogen levels by secreting β-glucuronidase (GUS), an enzyme that converts conjugated estrogen into deconjugated estrogen in the gastrointestinal tract. This transformation allows it to bind to estrogen receptors, initiating downstream signaling and physiological effects[10,11]. In the lower female reproductive tract, estrogen regulates the microenvironment by increasing epithelial thickness, glycogen levels, mucus secretion, and indirectly lowering vaginal pH through the promotion of Lactobacilli abundance and lactic acid production[12]. Additionally, estrogen can modify gut epithelial barrier integrity[13].
Decreased GUS activity may lead to reduced deconjugation of estrogen, resulting in decreased circulating estrogen levels and contributing to pathologies such as obesity and polycystic ovarian syndrome (PCOS). In contrast, increased GUS activity can elevate estrogen levels, leading to conditions such as endometriosis and cancer[14,15]. Endogenous estrogen is a major factor in the development of postmenopausal breast cancer. Certain bacterial genera and species in the human gut, including Bacteroides, Escherichia, and Lactobacillus, contain genes encoding GUS[16]. In a study on mice with letrozole-induced PCOS, serum estradiol levels positively correlated with the abundance of Bifidobacterium and Bacteroides, while negatively correlating with the abundance of Prevotella[17]. The investigation by Shin et al[18] on 26 healthy women revealed that those in the high-estradiol group harbored a more diverse gut microbiota compared to the low- and medium-estradiol groups. The drop in serum estradiol concentration was attributed to the relative overabundance of Slackia and Butyricimonas[18]. Another study found a significant and positive association between non-ovarian urine estrogen levels and Clostridia taxa in the Firmicutes (including non-Clostridiales and three genera in the family Ruminococcaceae)[19].
Through the breakdown of organic matter, gut microbiota produces metabolites such as short-chain fatty acids (SCFA) and bile acids. SCFAs, including acetic acid, propionic acid, and butyric acid, provide energy for colon cells, regulate the intestinal barrier, and influence the inflammatory response[20-23]. Firmicutes predominantly synthesize butyrate[24], while Bacteroides are major producers of acetate and propionate[25]. Butyric acid has been shown to regulate progesterone and estradiol secretion through the cAMP signaling pathway in porcine granulosa cells[26]. The study by Liu et al[27] demonstrated that supplementing with butyrate can alleviate nonalcoholic fatty liver disease in ovariectomized mice. One study found that SCFAs have anti-inflammatory properties mediated through the G protein-coupled receptor pathway and histone acetylase[28]. Notably, SCAFs may have anti-cancer properties in cervical cancer through the activation of free fatty acid receptor 2[29].
Bile acid plays an important role in maintaining intestinal homeostasis, regulating lipid and carbohydrate metabolism, and influencing immune function. The study by Qi et al[30] reported that elevated levels of Bacteroides vulgatus were observed in the gut microbiota of individuals with PCOS, and this elevation was accompanied by reduced levels of glycodeoxycholic acid and tauroursodeoxycholic acid. The study found that glycodeoxycholic acid induced intestinal group 3 innate lymphoid cell IL-22 secretion through GATA binding protein 3. IL-22, in turn, improved the PCOS phenotype[30]. In an in vitro experiment, it was demonstrated that urolithin A reduces the Rac1 and PAK1 activity, leading to a decrease in actin polymerization and consequently reducing cell migration in human endometrial carcinoma cells[31], urolithin A may offer new avenues for the development of novel cancer therapeutics.
PCOS is a prevalent endocrine disorder in women, characterized by symptoms such as anovulation, obesity, insulin resistance, and hyperandrogenism. Tremellen and Pearce[32] highlighted that disturbances in the gut microbiota resulting from a poor diet can lead to increased gut mucosal permeability. Subsequently, this allows LPS from Gram-negative colonic bacteria to enter the systemic circulation. The subsequent activation of the immune system interferes with insulin receptor function, elevating serum insulin levels, consequently contributing to increased androgen production by the ovaries and disruption of normal follicle development[32]. A study involving 18 obese patients with PCOS and 15 obese women without PCOS revealed that the richness and diversity of gut microbiota were lower in the obese PCOS group compared to the control group, Lachnoclostridium, Fusobacterium, Coprococcus_2, and Tyzzerela 4 were identified as the characteristic genera in obese patients with PCOS[33]. Additionally, mice transplanted with stool from women with PCOS exhibited fewer pups compared to mice transplanted with stool from healthy controls[30]. Modulating gut microbiota could hold significant value for the treatment of PCOS[34,35].
Endometriosis is a chronic inflammatory disease characterized by the presence of endometrial tissue (glands and stroma) outside the uterus, significantly impacting the quality of life of women of childbearing age[36]. Although endometriosis is known to be estrogen-dependent, studies have revealed that the growth of ectopic lesions persists even in ovariectomized animals. This suggests that in addition to ovarian steroids, the innate immune system of the pelvic environment can also regulate the growth of ectopic lesions in endometriosis[37]. Notably, gut microbiota dysbiosis has been associated with the occurrence and development of endometriosis. A study involving 14 women with histologically confirmed stage 3/4 endometriosis and 14 healthy controls demonstrated significant decreases in the genera Sneathia, Barnesella, and Gardnerella in stool samples of the endometriosis group[38]. Two patients in the endometriosis group exhibited higher levels of Escherichia/Shigella in stool, and subsequent follow-up revealed severe bowel involvement by endometriosis in these patients[38]. Moreover, a study using antibiotic-induced microbiota-depleted (MD) mice to investigate endometriosis progression demonstrated that MD mice exhibited reduced endometriotic lesion growth, and the transplantation of gut microbiota through oral gavage of feces from mice with endometriosis caused the endometriotic lesion growth[39]. Thus, these findings underscore the close relationship between the occurrence and development of endometriosis and gut microbiota.
Dysbiosis of microbiota can impact the occurrence and progression of tumors by regulating host immune response and inflammatory pathways. Breast cancer is one of the most prevalent malignant tumors among women worldwide and is closely linked to estrogen levels. The gut microbiota plays a role in deconjugating estrogens through the bacterial secretion of GUS, enabling estrogens to bind to estrogen receptors. Subsequently, the activation of estrogen receptors increases the number of G0/G1 cells entering the cell cycle, promoting cell proliferation, which is particularly well-defined in breast cancer[40]. The study conducted by Bobin-Dubigeon et al[41] demonstrated a reduction in gut microbiota diversity, a relative enrichment in Firmicutes, and a depletion in Bacteroidetes among patients with breast cancer as compared to those of healthy women. In another controlled study, patients with breast cancer exhibited a lower abundance of some microbial taxa, including Bacteroidetes phylum, Firmicutes phylum, Verrucomicrobia phylum, Clostridium genus, Shigella genus, Bifidobacterium genus, Akkermansia muciniphila, Clostridium perfringens, Escherichia coli, Bacteroides uniformis, Clostridium hathewayi, and Faecalibacterium prausnitzii[42].
Cervical cancer ranks as the fourth most common cancer in terms of morbidity and mortality, primarily attributed to human papillomavirus infection. In a study involving 42 patients with cervical cancer, gut microbiota 16S rDNA analysis revealed differences in both α and β diversity between the patient group and the control group[43]. The patient group exhibited a higher abundance of Prevotella, Porphyromonas, and Dialister, while the control group showed a higher abundance of Bacteroides, Alistipes, and members of the Lachnospiracea family[43]. Additionally, Chang et al[44] conducted an enrichment analysis of gut microbiota from patients with cervical cancer patients and healthy controls and found that the functions of the differentially expressed genes in the two groups, primarily associated with REDOX reactions, biosynthesis of other secondary metabolites, and amino acid transport and metabolism.
Ovarian cancer, with the highest mortality among female genital tract malignancies, is often diagnosed at advanced stages with metastasis. Endoscopic ultrasound is a non-invasive and accurate method for the early diagnosis of early carcinoma in the upper gastrointestinal tract and liver diseases[45,46]. Additionally, this tool is effective in detecting ovarian cancer infiltration of surrounding organs[47]. Xu et al[4] demonstrated in vitro that gut microbiota dysbiosis can promote the growth of ovarian cancer cells and induce epithelial-mesenchymal transition. This underscores the need for further exploration of the role of gut microbiota in the occurrence and development of various tumors. Table 1 summarizes the list of studies that highlighted the gut microbiota changes in patients with PCOS, endometriosis, breast cancer, cervical cancer, and ovarian cancer.
| Diseases | Changes in gut microbiota (human studies) |
| PCOS | Increase: Lachnoclostridium, Fusobacterium, Coprococcus_2, and Tyzzerela 4[33]; Bacteroides, Escherichia/Shigella, and Streptococcus[48] |
| Decrease: Tenericutes and Firmicutes/Bacteroides ratio[33]; Akkermansia and Ruminococcaceae[48] | |
| Endometriosis | Increase: Bacteroides, Parabacteroides Oscillospira, and Coprococccus[49] |
| Decrease: Paraprevotella and Lachnospira[49]; Clostridia Clostridiales, Lachnospiraceae Ruminococcus, Clostridiales, Lachnospiraceae, and Ruminococcaceae Ruminococcus[50] | |
| Breast cancer | Increase: Firmicutes, Clostridium cluster IV, and cluster XIVa[41] |
| Decrease: Bacteroidetes[41]; Bacteroidetes phylum, and Verrucomicrobia phylum[42] | |
| Cervical cancer | Increase: Prevotella, Porphyromonas, and Dialister[43]; Succinivibrio, Ruminococcus, Morganella, Shewanella, and Proteus[51] |
| Decrease: Bacteroides, Alistipes, and Lachnospiracea[43]; Phascolarctobacterium and Halomonas[51] | |
| Ovarian cancer | Increase: Proteobacteria[52] |
| Decrease: Actinobacteria, Bifidobacterium, and Coprococcus[52] |
Probiotics have emerged as a modulator of gut microbiota. Recently, probiotics have been successfully used in the regulation of disrupted gut microbiota and the improvement of diseases such as gestational diabetes mellitus (GDM) and endometriosis. In a prospective study involving 256 pregnant women randomized to receive probiotics or a placebo during the first trimester, the probiotic intervention resulted in a reduced incidence of GDM[53]. Additionally, oral administration of lactic acid bacteria was found to alleviate endometriosis-related pain[54]. In a study by He et al[34], a PCOS-induced rat model treated through letrozole treatment showed that a 4-wk strain intervention, particularly with Lactobacillus plantarum HL2, was protective against PCOS-like pathological changes in the ovaries. Prebiotics are defined as a nondigestible food ingredient that selectively stimulates the growth and/or activity of specific bacteria in the colon; thus, improving host health[55]. Prebiotics improve the balance of gut microbiota and produce various beneficial effects on the human host, such as improving insulin resistance[56] and regulating intestinal immunity[57].
The technology of fecal microbiota transplantation (FMT) has gradually matured and found applications in various complex intestinal diseases. However, there has been limited research on the use of FMT for the treatment of gynecological diseases. In a study, rats with PCOS were observed to have lower levels of Lactobacillus and Clostridium, and higher levels of Prevotella compared to control rats[35]. Following treatment with FMT and Lactobacillus from healthy rats, the abnormal estrous cycle improved, and androgen biosynthesis decreased in all rats in the FMT group and 75% of the rats in the Lactobacillus group. Moreover, ovarian morphology normalized, and the composition of the recovered gut microbiota in the FMT and Lactobacillus-treated groups demonstrated an increase in Lactobacillus and Clostridium and a decrease in Prevotella[35]. Huang et al[2] performed ovariectomy on 12-wk-old mice and subsequent follow-up revealed vaginal atrophy and disrupted intestinal microbial balance at 4 wk post-operation. Subsequent transplantation of gut microbiota from normal female mice to ovariectomized mice resulted in enhanced proliferation of vaginal epithelium and significant alleviation of epithelial atrophy. The abundance of bacteria positively influencing vaginal epithelial regeneration (Proteobacteria, Verrucomicrobia, Akkermansia) increased as observed in the study. Therefore, further research on FMT could offer a new alternative treatment for gynecological diseases.
The impact of gut microbiota on the body’s immune system and hormonal balance is significant, dysbiosis of gut microbiota has been associated with the promotion of common gynecological diseases, such as PCOS, endometriosis, and malignant tumors. Conversely, these diseases can further disrupt the balance of gut microbiota. Interventions targeting the imbalanced gut microbiota, including the use of probiotics, prebiotics, and FMT, have shown promising results in animal experimental models. This approach offers a new perspective on the treatment of gynecological diseases, although further clinical studies are necessary to validate these findings. In the future, exploring the possibility of “matching” FMT donors and recipients or using bioengineering to synthesize bacterial solutions for precise disease treatment is worth further exploration.
We would like to thank the Department of Gastroenterology and Endoscopic Center of Shengjing Hospital of China Medical University for technical assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 823] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 2. | Huang J, Shan W, Li F, Wang Z, Cheng J, Lu F, Guo E, Beejadhursing R, Xiao R, Liu C, Yang B, Li X, Fu Y, Xi L, Wang S, Ma D, Chen G, Sun C. Fecal microbiota transplantation mitigates vaginal atrophy in ovariectomized mice. Aging (Albany NY). 2021;13:7589-7607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Marano G, Traversi G, Gaetani E, Gasbarrini A, Mazza M. Gut microbiota in women: The secret of psychological and physical well-being. World J Gastroenterol. 2023;29:5945-5952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Reference Citation Analysis (2)] |
| 4. | Xu S, Liu Z, Lv M, Chen Y, Liu Y. Intestinal dysbiosis promotes epithelial-mesenchymal transition by activating tumor-associated macrophages in ovarian cancer. Pathog Dis. 2019;77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Jin J, Gao L, Zou X, Zhang Y, Zheng Z, Zhang X, Li J, Tian Z, Wang X, Gu J, Zhang C, Wu T, Wang Z, Zhang Q. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ Res. 2022;131:492-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 6. | Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 602] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 7. | Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740-E747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 725] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 8. | Arnone AA, Cook KL. Gut and Breast Microbiota as Endocrine Regulators of Hormone Receptor-positive Breast Cancer Risk and Therapy Response. Endocrinology. 2022;164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 676] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Chadchan SB, Singh V, Kommagani R. Female reproductive dysfunctions and the gut microbiota. J Mol Endocrinol. 2022;69:R81-R94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021;28:92-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 12. | Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:G466-G472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 481] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 15. | Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 553] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 16. | Kwa M, Plottel CS, Blaser MJ, Adams S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 17. | Wu YX, Yang XY, Han BS, Hu YY, An T, Lv BH, Lian J, Wang TY, Bao XL, Gao L, Jiang GJ. Naringenin regulates gut microbiota and SIRT1/ PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed Pharmacother. 2022;153:113286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 19. | Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 406] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 20. | Ziętek M, Celewicz Z, Szczuko M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 21. | Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 1178] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 22. | Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 356] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016;7:979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 979] [Cited by in RCA: 1085] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 24. | Gasaly N, Hermoso MA, Gotteland M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 25. | Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1299] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 26. | Lu N, Li M, Lei H, Jiang X, Tu W, Lu Y, Xia D. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. J Steroid Biochem Mol Biol. 2017;172:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Liu L, Fu Q, Li T, Shao K, Zhu X, Cong Y, Zhao X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS One. 2022;17:e0262855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Zhang Z, Zhang H, Chen T, Shi L, Wang D, Tang D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun Signal. 2022;20:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 29. | Matsuya-Ogawa M, Shibata T, Itoh H, Murakami H, Yaguchi C, Sugihara K, Kanayama N. Oncoprotective Effects of Short-Chain Fatty Acids on Uterine Cervical Neoplasia. Nutr Cancer. 2019;71:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y, Jiang C, Qiao J. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 497] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 31. | Alauddin M, Okumura T, Rajaxavier J, Khozooei S, Pöschel S, Takeda S, Singh Y, Brucker SY, Wallwiener D, Koch A, Salker MS. Gut Bacterial Metabolite Urolithin A Decreases Actin Polymerization and Migration in Cancer Cells. Mol Nutr Food Res. 2020;64:e1900390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Zhou L, Ni Z, Yu J, Cheng W, Cai Z, Yu C. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2020;11:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | He Y, Wang Q, Li X, Wang G, Zhao J, Zhang H, Chen W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020;11:5192-5204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L. Association between Polycystic Ovary Syndrome and Gut Microbiota. PLoS One. 2016;11:e0153196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 36. | Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 612] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Nicholes K, Shih IM. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol. 2020;15:71-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 38. | Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, Urman B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci Rep. 2019;9:2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 39. | Chadchan SB, Naik SK, Popli P, Talwar C, Putluri S, Ambati CR, Lint MA, Kau AL, Stallings CL, Kommagani R. Gut microbiota and microbiota-derived metabolites promotes endometriosis. Cell Death Discov. 2023;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA, Sutherland RL. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer. 2003;10:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Bobin-Dubigeon C, Luu HT, Leuillet S, Lavergne SN, Carton T, Le Vacon F, Michel C, Nazih H, Bard JM. Faecal Microbiota Composition Varies between Patients with Breast Cancer and Healthy Women: A Comparative Case-Control Study. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Caleça T, Ribeiro P, Vitorino M, Menezes M, Sampaio-Alves M, Mendes AD, Vicente R, Negreiros I, Faria A, Costa DA. Breast Cancer Survivors and Healthy Women: Could Gut Microbiota Make a Difference?-"BiotaCancerSurvivors": A Case-Control Study. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 43. | Sims TT, Colbert LE, Zheng J, Delgado Medrano AY, Hoffman KL, Ramondetta L, Jazaeri A, Jhingran A, Schmeler KM, Daniel CR, Klopp A. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecol Oncol. 2019;155:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Chang L, Qiu L, Lei N, Zhou J, Guo R, Gao F, Dong S, Chen M, Wu F, Qin B. Characterization of fecal microbiota in cervical cancer patients associated with tumor stage and prognosis. Front Cell Infect Microbiol. 2023;13:1145950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 45. | Chen H, Wang X, Shao S, Zhang J, Tan X, Chen W. Value of EUS in determining infiltration depth of early carcinoma and associated precancerous lesions in the upper gastrointestinal tract. Endosc Ultrasound. 2022;11:503-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Jearth V, Sundaram S, Rana SS. Diagnostic and interventional EUS in hepatology: An updated review. Endosc Ultrasound. 2022;11:355-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Carvalho J Jr, Formighieri B, Filippi S, Rossini L. Location of recurrent asymptomatic ovarian cancer through endoscopic ultrasound. Endosc Ultrasound. 2015;4:63-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, Ling Y, Fu H, Dong W, Shen J, Reeves A, Greenberg AS, Zhao L, Peng Y, Ding X. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front Microbiol. 2017;8:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 49. | Svensson A, Brunkwall L, Roth B, Orho-Melander M, Ohlsson B. Associations Between Endometriosis and Gut Microbiota. Reprod Sci. 2021;28:2367-2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 50. | Huang L, Liu B, Liu Z, Feng W, Liu M, Wang Y, Peng D, Fu X, Zhu H, Cui Z, Xie L, Ma Y. Gut Microbiota Exceeds Cervical Microbiota for Early Diagnosis of Endometriosis. Front Cell Infect Microbiol. 2021;11:788836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Wang Z, Wang Q, Zhao J, Gong L, Zhang Y, Wang X, Yuan Z. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express. 2019;9:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Hu X, Xu X, Zeng X, Jin R, Wang S, Jiang H, Tang Y, Chen G, Wei J, Chen T, Chen Q. Gut microbiota dysbiosis promotes the development of epithelial ovarian cancer via regulating Hedgehog signaling pathway. Gut Microbes. 2023;15:2221093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 53. | Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 54. | Khodaverdi S, Mohammadbeigi R, Khaledi M, Mesdaghinia L, Sharifzadeh F, Nasiripour S, Gorginzadeh M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int J Fertil Steril. 2019;13:178-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 55. | Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G Jr, Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME. Prebiotics: why definitions matter. Curr Opin Biotechnol. 2016;37:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 56. | Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 57. | Martyniak A, Medyńska-Przęczek A, Wędrychowicz A, Skoczeń S, Tomasik PJ. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |