Copyright
©The Author(s) 2024.
World J Gastroenterol. Jan 7, 2024; 30(1): 34-49
Published online Jan 7, 2024. doi: 10.3748/wjg.v30.i1.34
Published online Jan 7, 2024. doi: 10.3748/wjg.v30.i1.34
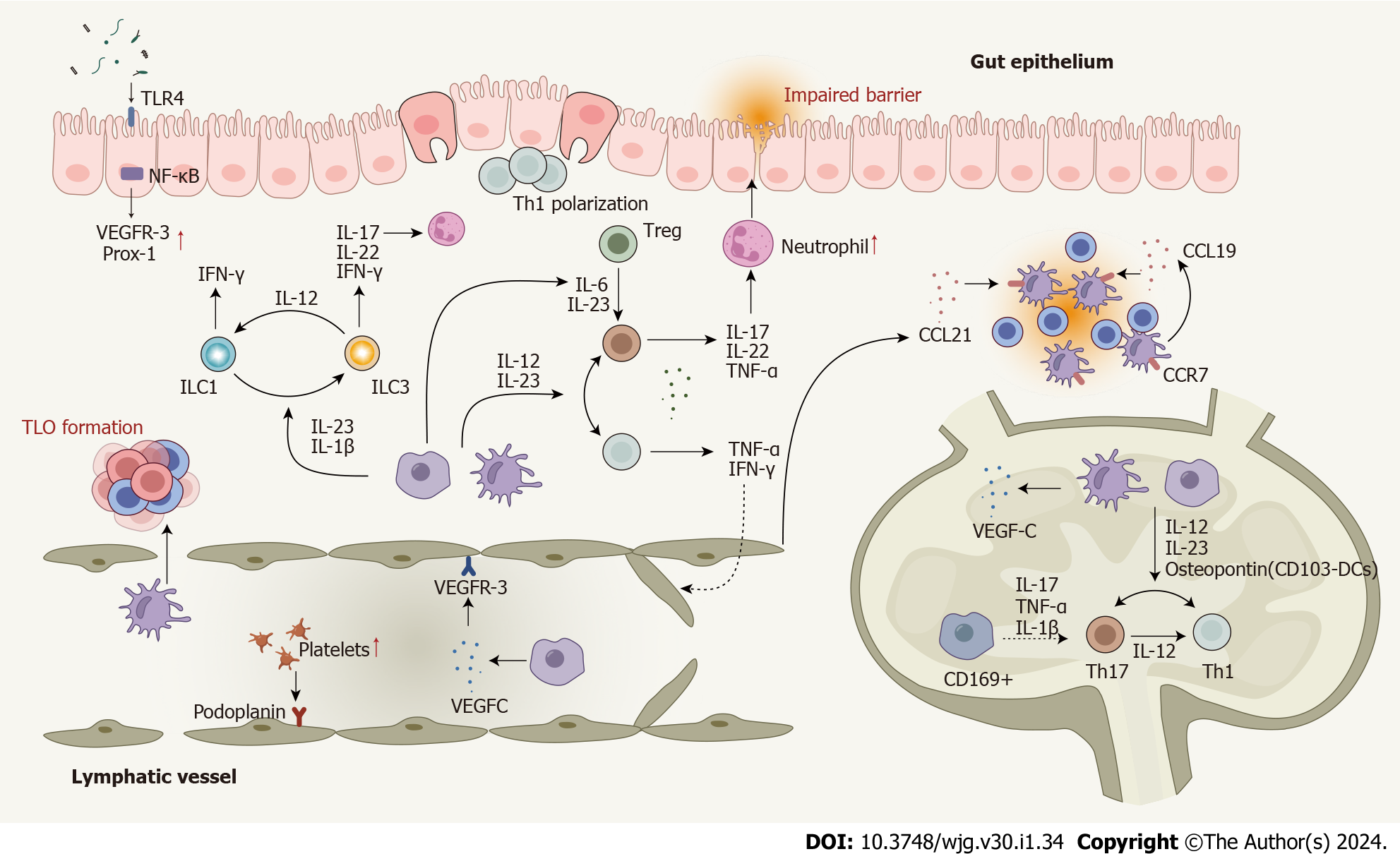
Figure 3 Dysregulated cells, cytokines, and enhanced cell plasticity in the lymphatic system in Crohn’s disease.
T cells are polarized to Th1 and Th17 cells in response to pro-inflammatory cytokines produced by macrophages and dendritic cells (DCs). Several cytokines play roles in Th17-Th1 and Treg-Th17 trans-differentiation. DCs, macrophages, T cells, and B cells contribute to lymphangiogenesis by mediating the expression of lymphangiogenic factors. Impaired lymphatic function and dysregulated chemokines lead mature DCs and T cells away from their usual mesenteric lymph nodes. Innate lymphoid cells (ILCs) also contribute to Crohn’s disease (CD) pathogenesis and enhanced plasticity between ILC1 and ILC3 has been observed. Several signaling pathways involved in the lymphatic system actively participate in the CD pathogenesis. Nuclear factor-kappa B is involved in lymphatic remodeling by directly up-regulating the expression of VEGFR-3 and Prox-1. The mTOR signaling affects leukocyte trafficking through the lymphatic barrier in CD. CD: Crohn’s disease; DC: Dendritic cells; IFN: Interferon; IL: Interleukin; ILC: Innate lymphoid cell; NF-κB: Nuclear factor-kappa B; Prox-1: Prospero homeobox protein 1; Th: T helper; TLO: Tertiary lymphoid organ; TLR4: Toll-like receptor 4; TNF: Tumor necrosis factor; Treg: Regulatory T cell; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; MAT: Mesenteric adipose tissue.
- Citation: Zhou YW, Ren Y, Lu MM, Xu LL, Cheng WX, Zhang MM, Ding LP, Chen D, Gao JG, Du J, Jin CL, Chen CX, Li YF, Cheng T, Jiang PL, Yang YD, Qian PX, Xu PF, Jin X. Crohn’s disease as the intestinal manifestation of pan-lymphatic dysfunction: An exploratory proposal based on basic and clinical data. World J Gastroenterol 2024; 30(1): 34-49
- URL: https://www.wjgnet.com/1007-9327/full/v30/i1/34.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i1.34