Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.147
Revised: June 13, 1996
Accepted: July 20, 1996
Published online: September 15, 1997
AIM: To investigate the source of the blood supply in carvenous hemangioma of liver (CHL), and provide a feasible treatment for CHL via thehepatic artery.
METHODS: (1) Portovenography, hepatic arteriography and portal vein staining were performed in 5 patients to determine the origin of the blood supply. Two casts of hepatic blood vessels from resected specimens were observed. (2) Clinical data from 75 patients (30 males, 45 females, aged 25-57 years, mean of 37.4) were obtained. Of these, 56 were of solitary type (44 on the right lobe, 12 on the left, with 4 having intraparenchyma), and 19 were of multiple type (9 on the right, 2 the left, 8 whole liver). Twenty-two patients were treated with sclerosis, 50 by embolization via hepatic artery, and 3 were excised.
RESULTS: In the 5 cases where portography was used, the contrast medium did not enter the tumor, and the tumor appeared as low density area, with the intrahepatic branches of the portal vein pushed aside. In the 5 cases with where portal vein staining was used, the normal liver parenchyma stained a deep blue; however, the tumor was not stained. The tumor area appeared as a round vacant cavity in the 2 specimen casts. For the 72 patients treated with sclerosis or embolization via hepatic artery or through interventional method, the tumors diminished by 10%-30% in diameter, and no tumors grew larger.
CONCLUSION: The blood supply of CHL originates from the hepatic artery. Tumors treated with sclerosis and embolization decreased in size or got fibrotic.
- Citation: Li GW, Zhao ZR, Li BS, Liu XG, Wang ZL, Liu QF. Source of blood supply and embolization treatment in cavernous hemangioma and sclerosis of the liver. World J Gastroenterol 1997; 3(3): 147-149
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/147.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.147
Cavernous hemangioma of the liver (CHL) is a common, benign, hepatic tumor. With the development and wide application of modern imaging technics, many more cases of CHL have been diagnosed in recent years. Currently, the conventional treatment for CHL is excision of the tumor. In this report, we studied the blood supply of CHL from December 1985 to November 1992, and we found that CHL is a malformation of hepatic arterial vasculature. In this study, 22 of 72 CHL patients were treated using sclerosis, while the other 50 were treated with gelfoam microspheres with lipiodol administered either during surgery or using hepatic arterial intubation via the femoral artery. In all cases, the results were satisfactory.
The portovenography and hepatic arteriography were performed in five patients as previously reported[1].
In these five cases, the portal veins were intubated via the right gastroepiploic vein in the right gastric vein, or via direct puncture of the portal vein, and 60 mg of methylene blue diluted to a final volume of 20 mL was quickly injected. The staining of the liver parenchyma and the tumor body were then observed.
The two resected CHL specimens from left lobes, 10 cm and 10.5 cm in diameter, were fixed with formalin and the sections were sent for serial pathological observations. Another resected specimen, 6 cm in diameter, was made into model cast by filling the hepatic vein (yellow) and portal venous branch (blue) with methyl methacrylate after vascular lavation.
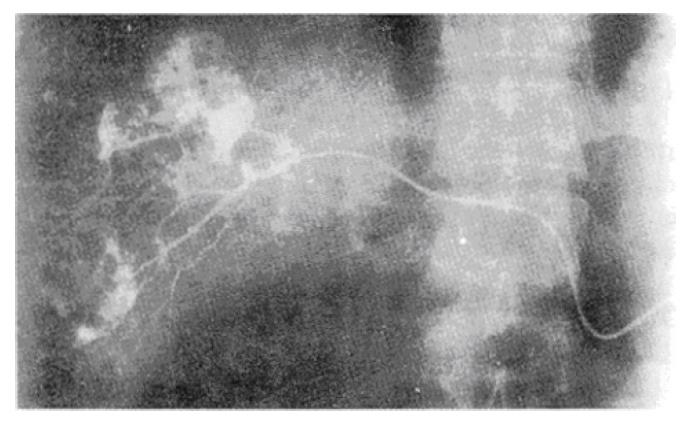
In the 5 cases with in which portography was used, the contrast medium could not enter the tumor, and the intrahepatic branches of portal vein were pushed aside by the tumor. During the liver parenchymal phase-contrast, the density of the normal tissue was obviously increased, while the tumor appeared to be of lower density, with sharp and clear boundaries. In 22 patients that underwent sclerosing therapy, hepatic arteriography revealed that the contrast medium immediately entered the tumor, and had a “cotton” or “popcorn” appearance. In these patients, an arteriovenous fistula was not observed (Figures 1 and 2).
After the injection of methylene blue through portal vein, the normal liver parenchyma was stained homogenously in a deep blue; however, the tumor was not appear stained, and the boundary between the normal liver tissue and the tumor was sharp (Figure 3).
In the serial sections of nine biopsies and resected specimens, no normal blood vessels or bile ducts were observed under microscopy. Although CHL did not have a capsule, it did have a clear boundary with the neighboring normal liver tissues.
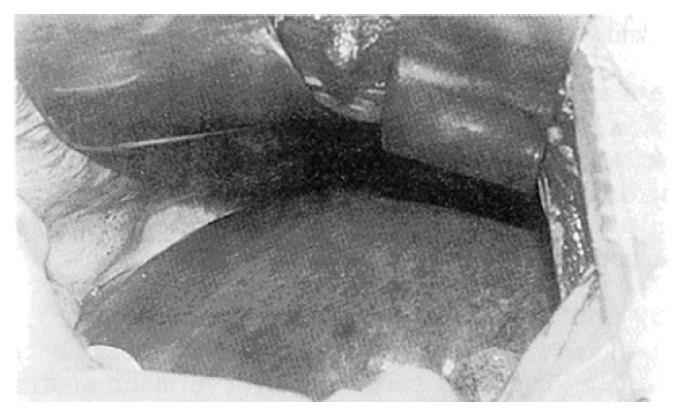
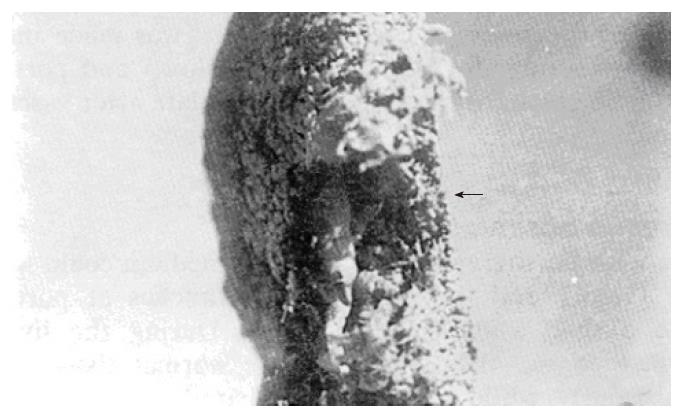
The casting specimens showed that the eroded tumor left behind a round vacant area in the specimens. The stained branches of the portal vein did not extend into the tumor (Figure 4).
Among the 75 patients included in this study, 30 were males and 45 females, with an age ranged between 25-57 years, with a mean age of 37.4 years. All patients were diagnosed by ultrasound, with 66 confirmed by CT, 34 by selective coeliac arteriography, and 20 by ECT. The diameter of the CHL ranged 6-30 cm, with only 4 of them greater than 20 cm. Among these 75 patients, 56 cases were of solitary type, with 44 were on the right lobe, 12 on the left and 4 were intraparenchymatous. Nineteen of the 75 patients had multiple type, with 9 on the right, 2 on the left and 8 in the whole liver. Of the original 75 cases, three cases were excised; the left hepatic artery was not shown on arteriography for 2 of them, however, but were confirmed during surgery. In the other 72 cases, 22 received sclerosing therapy, and 50 were treated with embolization (10 of these were interventional embolizations). Among the 75 cases, 2 were HBsAg positive, 1 HBsAb positive and none of them had cirrhosis. Twenty-one were proved to be CHL pathologically.
Under direct view, the involved hepatic arterial branches were dissected and confirmed by methylene blue injection. The proximal end was ligated and intubated through the distal end, and the tube was then fixed with rubber pieces. The opening of the tube was filled with normal saline and fire sealed, before being pulled out through the abdominal wall puncture, and fixing an adhesive plaster on the skin. Through this tube, sclerosing treatment and repeated radiographic examinations were carried out[1].
For the sclerosing therapy group, the patients were divided into three subgroups. Subgroup 1 (n = 8) had 10-20 mL of 40% carbamide injected through the intubated arterial branch of the liver once daily for 20-30 d. Subgroup 2 (n = 6) had 10 mL of 49.9% ethanol injected by the same route, once every three days, for a total of 5-7 injections. Subgroup 3 (n = 8) had 2 carbamide injections and one ethanol injection administered alternatively, with usually 9 injections required.
For the embolization therapy group, 100 mg of gelfoam particles mixed with 8-16 mL of lipiodol emulsion was injected. Ten patients were given interventional embolization. Generally, one single embolization was adequate. It was only when the tumor diameter was greater than 10 cm that a second embolization was needed. In cases with multiple tumors less than 4 cm in diameter on the other lobe of the liver, anhydrated ethanol was injected into the tumor at multiple points. The injection was stopped when the tumor appeared blanched or collapsed. After withdrawal of the needle, finger pressing was used to prevent bleeding and extravasation of ethanol.
In the sclerosing therapy group, periodical radiography was performed. The tube was pulled out until the tumor body could not be visualized. The embolization treatment was discontinued when the tumor had shrunk by 10% based on X-ray evaluation or after two-weeks or one month. When the diameter was beyond 10 cm, a second embolization was given. Among the 28 cases, a total of 36 embolizations were performed.
(1) On intensified CT scanning, the “ring sign” and “half ring sign” reduced in size or disappeared totally after 3-6 mo. (2)The dynamic isotopic hepatic blood pool scanning showed a radioactive defect. And (3) Shrinkage of tumor on ultrasonography.
The carbamide in sclerosing therapy was not irritative, and usually it took 25 d to obliterate the CHL. Ethanol injection, however, elicited intolerable pain, which lasted for about 2 h, and generally required seven injections. Interventional embolization produced lasting pain, which often required pethidine; however, only 1-2 times were needed, and the pain attenuated on the second treatment.
The 22 cases with sclerosing therapy were followed for 1-6 years, and CT and ultrasound examinations indicated the diameter of the tumor had shrunk by 20%-60%. In one patient who received a second operation, after one year, white and shining fibrous tissues were evident, which were confirmed pathologically. The other two female patients with tumors beyond 20 cm and who had previously had repeated skin petechiae, gained more than 5 kg of weight, and the petchiae disappeared after the treatment. The embolization group were followed for over six months, and the tumors for this group all diminished by 10%-30% in diameter, and none of the tumors grew any larger.
Since the beginning of this century, CHL has been regarded as a portal venous malformation by many researchers, but ligation of branches of portal vein was unsatisfactory as a treatment[2-5]. Yamamoto was the first to determine that the morphology of sinusoids of CHL specimens were different from that of portal and hepatic vein, but similar to that of hepatic artery using electron microscopy[6]. At present, a shunt for cavernous hemangioma has not been identified. Based on the portovenographic findings, intubational imaging of hepatic arterial branch, liver staining, and observations of the cast vessels in the resected specimens, this study has demonstrated that CHL is a malformation of the hepatic artery, and its blood supply arises completely from the hepatic artery without an arteriovenous shunt. This provides the theoretical basis for treating CHL via the hepatic artery. This study found that both sclerosing and embolization therapy can shrink the tumor and replace it with fibrotic tissues.
Hepatic cavernous hemangioma can grow continuously. Previously, Niemann reported 55 cases that led to diffuse hemangioma in the liver, which easily ruptured, causing a mortality rate as high as 70%[4,7]. However, excision is difficult when the tumor is of the multiple type, especially when it is near the hilum, very large in size, occupying the whole lobe of the liver, or some other contraindication exists. Our experience has found that therapy via intubation of hepatic artery is safe, reliable, and causes less injury, and does not require a blood transfusion.
Sclerosing agents, such as carbamide and ethanol, both require a long duration of therapy, especially the carbamide. Ethanol often produces pain for a brief period. However, Lipiodol can selectively stay for a long time in both benign and malignant hepatic tumors with rich blood supply. In this study, all 28 patients that received embolization therapy demonstrated such characteristics. Lipiodol and gelfoam microspheres are both long acting embolization agents. Gelfoam microspheres can occlude the vessels by secondary inflammation and growth of granulation tissues. The microspheres still could be identified in a few areas under microscopy after 28 d, and the hemangioma was completely filled with granulation tissues and fibrous tissues[7].
CHL is composed of cavernous blood sinusoids, without blood vessels, bile ducts or hepatocytes. Its blood supply primarily came from hepatic artery. The contrast agent and radioactive isotopes could quickly enter the periphery of the tumor, but diffused slowly and drained out after a long period, which accounts for the variegated feature of CHL. For example, the early visualization and late disappearance in hepatic angiography; the ring and half-ring signs on CT scanning, and the intensified imaging of blood pool radioactive scanning, is due to the fact that there are no hepatocytes within the tumor, making it appear that there is a radioactive defect in the static scanning. The above characteristics can also be the basis to assess the efficacy of occlusion treatment.
The variation of the hepatic artery is as high as 45%, particularly in the left lobe. This suggests that selective hepatic arteriography should be performed before intubation in order to raise the success rate of embolization therapy. Furthermore, interventional embolization therapy is only possible for certain patients. Since the hepatic artery tapers gradually, non-selective hepatic arteriography cannot show a complete picture of large and multiple hemangiomas, but rather requires superselective hepatic arteriography for the diagnosis and treatment of CHL.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Li GW, Liu XG, Li BSH, Wang ZL, Lei XY, Wang Y. Study on sclerosing therapy of hemangioma of the liver. Chinese Journal of Experimental Surgery. 1992;9:1-3. [Cited in This Article: ] |
| 2. | Kato M, Sugawara I, Okada A, Kuwata K, Satani M. Hemangioma of the liver. Diagnosis with combined use of laparoscopy and hepatic arteriography. Am J Surg. 1975;129:698-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Chao RS, Liu YF, Tian YS, He SG, Sheng K. Excision of cavernous hemangioma of the liver with decollement extra capsule. J Practical Surg. 1989;9:268-269. [Cited in This Article: ] |
| 4. | Li GC, Gu JZ. Child hemangioma. 1st ed. Shaanxi: Shaanxi Science and Technology Publication House 1991; 83-87. [Cited in This Article: ] |
| 5. | Wakabyasshi Y. Ligation of branches of portal vein against massive hemangioma of the right hepatic lobe. Jpn J Gastroenterol. 1966;63:245-249. [Cited in This Article: ] |
| 6. | Yamamoto K, Itoshima T, Ito T, Ukida M, Ogawa H, Kitadai M, Hattori S, Mizutani S, Nagashima H. Scanning electron microscopy of a liver cavernous hemangioma. Gastroenterol Jpn. 1983;18:15-20. [PubMed] [Cited in This Article: ] |
| 7. | Chen XL, Wang DQ, Zhong DC, Gua YS, Yang CH, Li XW. Study of embolizing hepatic artery with gelfoam microsphere. Chinese Journal of Experimental Surgery. 1991;8:166-167. [Cited in This Article: ] |