Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.137
Revised: October 20, 1996
Accepted: November 10, 1996
Published online: September 15, 1997
AIM: To investigate the role of endoscopic monitoring in small bowel transplantation.
METHODS: This study was conducted in two parts—an initial experimental study followed by a clinical study. In the experimental study, segmental small bowel allotransplantation was performed on white outbred pigs. Stomas were created for exteriorization of the proximal and distal ends of the intestines (Thiry-Vella loop). The grafts were monitored by endoscopy via stomas, with or without immunosuppressive therapy. For the clinical study, the whole small-bowel allograft of a woman with short bowel syndrome was endoscopically monitored via distal stoma.
RESULTS: The most common endoscopic findings of graft rejection following small bowel allotransplantation were mucosal erythema, erosion, and ulceration. Diffuse ulceration with bleeding occurred in the late phase of rejection.
CONCLUSION: Endoscopic monitoring is essential to small bowel transplantation.
- Citation: Li YS, Li JS, Li N, Jiang ZW, Li YX, Li XH. Endoscopic monitoring in small bowel transplantation. World J Gastroenterol 1997; 3(3): 137-138
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.137
Graft rejection and infection are important causes of failure following small bowel transplantation (SBT). Endoscopy of lesions and biopsy of graft are currently the most important modalities for the detection of signs of rejection or infections such as cytomegalovirus (CMV) infection. In this study, we investigate the role of endoscopic monitoring in SBT by a combined approach of both experimental and clinical studies.
Animals and experimental groups Twelve white outbred pigs weighing 18.5-22.5 kg were used in this study. General anesthesia was induced by administration of intravenous sodium pentobarbital. The animals were divided into two equal groups according to the presence or absence of immunosuppressive treatment: group I (n = 6) with immunosuppression (cyclosporine, Tripterygium wilfordii, and methylprednisolone); and group II (n = 6) without immunosuppression.
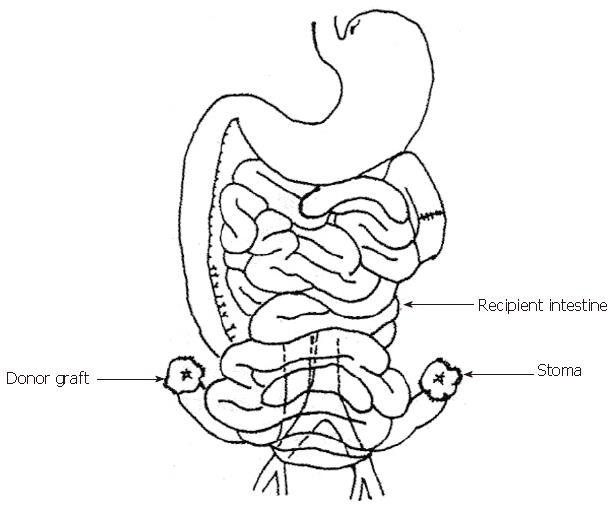
Heterotopic segmental small bowel allotransplantation was performed as described previously[1]. The graft was perfused with and preserved in Ringer′s lactate solution at 4 °C, followed by luminal perfusion with metronidazole solution at 4 °C. After a mean period of cold ischemia for 60 min, the segmental graft was vascularized. Both proximal and distal ends were exteriorized as stomas (Thiry-Vella loop) (Figure 1).
After the transplant, the grafts were evaluated daily with endoscopic visualization and biopsy, and the biopsy specimens were examined under a standard light microscopy.
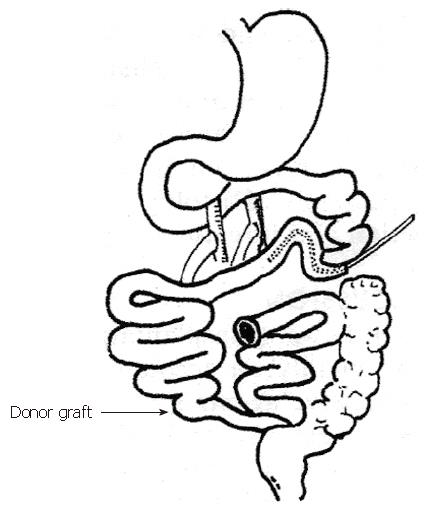
A woman with short bowel syndrome underwent whole small-bowel allotransplant (250 cm). The graft was orthotopically transplanted via end to side anastomosis of superior mesenteric artery aorta and end to side anastomosis of portal vein to superior mesenteric vein (Figure 2). Cyclosporine, Tripterygium wilfordii, and methylprednisolone were used as immunosuppressive agents. Biopsies guided or not guided by endoscopy were performed daily for 2 wk after the operation. From the 3rd to the 4th week, biopsies were performed every other or the third day. One month after the transplantation, endoscopy with biopsy was repeated. The biopsy specimens were examined under standard light microscopy.
In group I, all animals had survived a long period of time, while those in group II had a survival period of 17.7 ± 7.6 d. The cause of death in fatal cases was graft rejection. Thirty minutes after reperfusion, graft mucosal hyperemia and edema were seen. Mucosal erosion was seen on the first postoperative day (POD). Endoscopic and histologic features in the first 48 h were similar in both groups. Thereafter, in group I, graft mucosa was restored to normal appearance, except for occasional mucosal erosion and ulceration. On the other hand, group II showed punctate erythema on POD 4 or 5, local erosion or ulceration on POD 6 or 7, and diffuse ulceration with bleeding on POD 10. The histological features were necrosis and sloughing of villi tips, inflammatory cell infiltration, and intimal thickening or occlusion of vessels in submucosa.
From the 6th to the 8th month after the operation, eight endoscopic procedures with biopsies were performed. The endoscopic findings were graft mucosal hyperemia and edema on POD 6. Thereafter, endoscopic visualization showed absence of mucosal edema, glistening of the appearance of mucosa, presence of a normal vascular pattern, and increased peristalsis. Local ulceration with exudates was observed in the 3rd and the 5th month, respectively. Histological characteristics of graft rejection and infection were absent.
Small bowel transplantation has become a clinical reality mainly because of the availability of highly effective immunosuppressive agents such as cyclosporine, FK506, and OKT3. However, control of post-transplant complications such as graft rejection and infection and other complications following SBT continues to remain challenging. Signs of rejection and infection have been traditionally monitored using the absorptive function test and evaluation of motor activity, permeability of bowel, and levels of various chemical markers. These methods, however, could not entirely replace endoscopy with biopsy, and histological evaluation of biopsy continues to remain the most reliable investigation for confirming small intestinal allograft rejection[1,2].
In the clinical case of SBT, most of the operative procedures were similar to those in the experimental study (Figure 2). The graft distal stoma was used as the “window” for monitoring. Biopsy under naked eyes was slightly stimulative to the patients, but it was difficult to determine whether the specimens showed evidence of graft rejection. Specimens collected from sites adjacent to the stoma (< 10 cm) often showed signs of chronic inflammation, which are sometimes difficult to differentiate from those of rejection. Specimens obtained from the same site affected the diagnosis of rejection. In the clinical case of SBT, two biopsy samples taken from the colon did not show evidence of rejection, while two other biopsy samples observed under naked eyes showed characteristics suggestive of rejection histologically, but, endoscopically guided biopsies confirmed that rejection had not occurred. During the early stage of rejection, the mucosa shows local ulcer. Vascular characteristics were highly valuable in determining whether rejection has occurred. Therefore, it is important to perform full-thickness biopsies at several sites[1].
The rate of graft rejection was 75% in the first month and 15% for the first three months[3]. Abu-Elmagd et al[3] recommended that endoscopy should be performed weekly during the first three postoperative months. The results of our experimental study showed that early mucosal injury was largely related to reperfusion injury and that early definitive appearances of graft rejection were present on POD 5 or POD 6. Therefore, endoscopic visualization and biopsies of graft should commence on the 5th POD. In addition to routine regular endoscopic visualization, the endoscopic procedure should be repeated if any symptoms of rejection develop, including fever, abdominal pain, nausea, vomiting, watery diarrhea, increase in stromal output,, and dusking of mucosa. In the study by Abu Elmagd et al[3], the endoscopic appearances during acute rejection episode were ischemia or duskiness, local ulcer, and decrease or complete loss of peristalsis. Grafts with severe rejection showed diffuse ulceration with bleeding. Chronic graft rejection was evidenced by pseudomembrane formation, thickening of mucosal folds, and chronic ulceration. Hassanein et al[2] evaluated more than 220 endoscopic procedures and reported that during acute rejection mucosal features were mucosal erythema (77.8%), erosion (38%), and ulceration (33%). CMV infection appeared as local ulceration. In this study, graft rejection did not occur in the clinical case of SBT, and, the results of the experimental endoscopic visualization for signs of acute graft rejection were similar to those reported by Abu Elmagd et al[3] and Hassanein et al[2].
The findings of this study highlight the importance of endoscopic visualization with biopsy in the monitoring of cases of SBT for signs of graft rejection. However, the criteria for small intestinal graft rejection are yet to be definitively established, and the differentiation between rejection and CMV infection remains difficult. Mucosal erosion and ulceration are not characteristic of small intestinal graft rejection. However, in this study, mucosal ulceration was observed in the 3rd and 5th postoperative month in clinical SBT, but no clinical findings or histological features of graft rejection were observed. Because of the patchy distribution of the evidences of rejection, we recommend that multiple endoscopic visualization and biopsies be repeated at different sites for thorough evaluation.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Li YS, Li JS, Li N, Liao CX, Wu XH, Li NY. Histological and ultrastructural changes of segmental small bowel transplantation in the swine. Bulletin of Jinling Hospital. 1994;7:53-56. [Cited in This Article: ] |
| 2. | Hassanein T, Schade RR, Soldevilla-Pico C, Tabasco-Minguillan J, Abu-Elmagd K, Furukawa H, Kadry Z, Demetris A, Tzakis A, Todo S. Endoscopy is essential for early detection of rejection in small bowel transplant recipients. Transplant Proc. 1994;26:1414-1415. [PubMed] [Cited in This Article: ] |
| 3. | Abu-Elmagd KM, Tzakis A, Todo S, Reyes J, Fung J, Nakamura K, Wright H, Furukawa H, Demetris J, Van Thiel DH. Monitoring and treatment of intestinal allograft rejection in humans. Transplant Proc. 1993;25:1202-1203. [PubMed] [Cited in This Article: ] |