Copyright
©The Author(s) 2023.
World J Gastroenterol. Nov 28, 2023; 29(44): 5907-5918
Published online Nov 28, 2023. doi: 10.3748/wjg.v29.i44.5907
Published online Nov 28, 2023. doi: 10.3748/wjg.v29.i44.5907
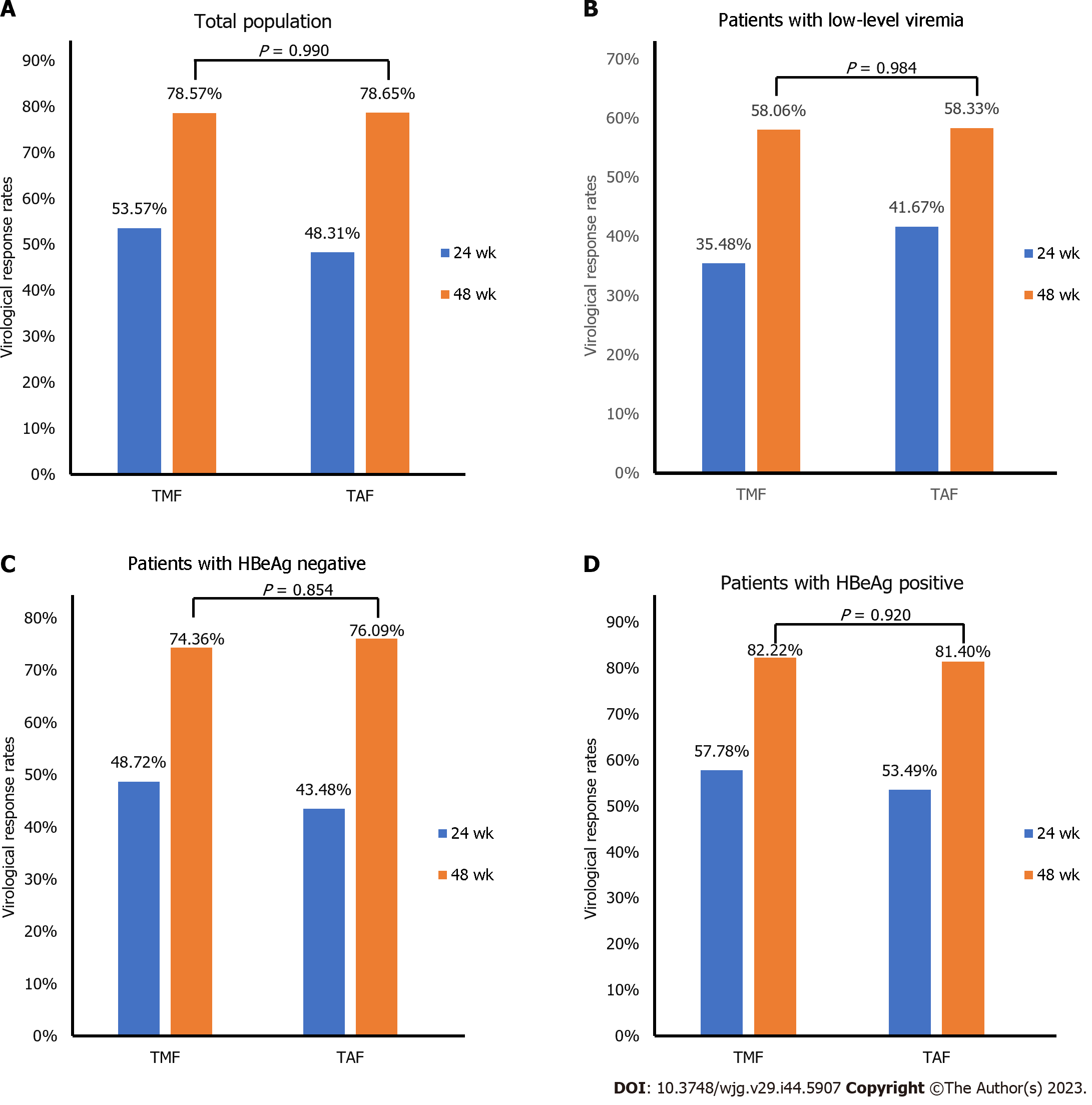
Figure 2 Comparison of virological response rates between tenofovir amibufenamide and tenofovir alafenamide.
A: Virological response (VR) rates of tenofovir amibufenamide (TMF) and tenofovir alafenamide (TAF) groups at 24 and 48 wk; B: VR rates of TMF and TAF groups at 24 and 48 wk with low-level viremia; C: VR rates of TMF and TAF groups at 24 and 48 wk with hepatitis B e antigen (HBeAg) negative; D: VR rates of TMF and TAF groups at 24 and 48 wk with HBeAg positive. TMF: Tenofovir amibufenamide; TAF: Tenofovir alafenamide; VR: Virological response; HBeAg: Hepatitis B e antigen.
- Citation: Peng WT, Jiang C, Yang FL, Zhou NQ, Chen KY, Liu JQ, Peng SF, Fu L. Tenofovir amibufenamide vs tenofovir alafenamide for treating chronic hepatitis B: A real-world study. World J Gastroenterol 2023; 29(44): 5907-5918
- URL: https://www.wjgnet.com/1007-9327/full/v29/i44/5907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i44.5907