Copyright
©The Author(s) 2023.
World J Gastroenterol. Oct 28, 2023; 29(40): 5593-5617
Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5593
Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5593
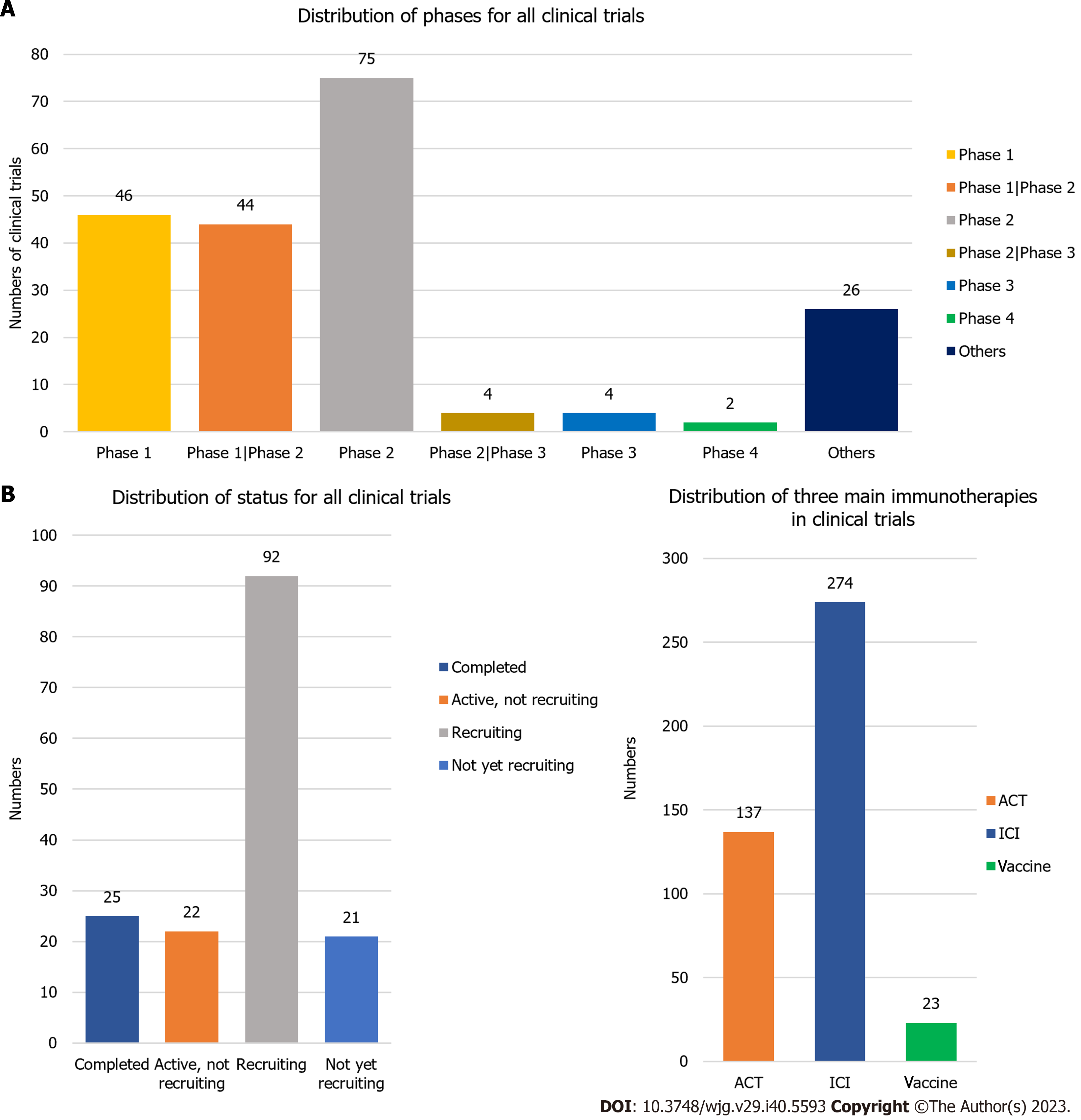
Figure 9 Phases and status distribution across all clinical trials.
A: Distribution of phases for all clinical trials. Phases include: Phase 1, phase 1/phase 2, phase 2, phase 2/phase 3, phase 3, phase 4, and others; B: Distribution of status for all clinical trials. The status includes: Completed, active but not recruiting, recruiting, and not yet recruiting; C: Distribution of adoptive cell therapy, immune checkpoint inhibitor, and vaccine in clinical trials. ICI: Immune checkpoint inhibitor; ACT: Adoptive cell therapy.
- Citation: Li YN, Xie B, Zhang Y, He MH, Xing Y, Mu DM, Wang H, Guo R. Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999-2023). World J Gastroenterol 2023; 29(40): 5593-5617
- URL: https://www.wjgnet.com/1007-9327/full/v29/i40/5593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i40.5593