Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5566
Peer-review started: August 14, 2023
First decision: September 19, 2023
Revised: October 4, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: October 28, 2023
Comprehensive genomic analysis has shown that small bowel adenocarcinoma (SBA) has different genomic profiles from gastric and colorectal cancers. Hence, it is essential to establish chemotherapeutic regimens based on SBA characteristics. The expression of programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 (PD-L2) in SBA is not fully understood. Anti-PD-L1/PD-1 therapy uses tumor-infiltrating lymphocytes (TILs); therefore, the status of TILs in the tumor microenvironment (TME) may influence their efficacy. The ratio of FoxP3+ to CD8+ T cells has been reported to be useful in predicting the prognosis of digestive system cancers.
To investigate the clinicopathological significance of PD-L1/2 expression according to the status of TILs in SBA tissues.
We performed immunohistochemical analysis for PD-L1, PD-L2, CD8, FoxP3, and DNA mismatch repair (MMR) proteins using formalin-fixed, paraffin-embedded tissues from 50 patients diagnosed with primary SBA. The immunoreactivities of PD-L1 and PD-L2 were determined separately in tumor cells and tumor-infiltrating immune cells throughout the tumor center and invasive margins, and finally evaluated using the combined positive score (CPS). We assessed CD8+ and FoxP3+ T cells in the intratumoral and tumor-surrounding stroma. Subsequently, we calculated and summed the ratio of FoxP3 to CD8+ T cell counts. Immune-related cell densities were graded as low or high. Immunohistochemical results were compared with clinicopathological factors and patient prognosis. The distribution of cancer-specific survival (CSS) was estimated using the Kaplan–Meier method, and the log-rank test was used to test for significant differences in CSS. A Cox proportional hazard model was also used to assess the effect of tumor variables on CSS.
PD-L1 expression was positive in 34% in tumor cells (T-PD-L1) and 54% in tumor-infiltrating immune cells (I-PD-L1) of the cases examined. T-PD-L2 was positive in 34% and I-PD-L2 was positive in 42% of the cases. PD-L1 CPS ≥ 10 and PD-L2 CPS ≥ 10 were observed in 50% and 56% of the cases, respectively. Deficient MMR (dMMR) was 14% of the cases. T-PD-L1, I-PD-L1 and PD-L1 CPS ≥ 10 were all significantly associated with dMMR (P = 0.037, P = 0.009, and P = 0.005, respectively). T-PD-L1, I-PD-L1, and PD-L1 CPS ≥ 10 were all associated with deeper depth of invasion (P = 0.001, P = 0.024, and P = 0.002, respectively). I-PD-L2 expression and PD-L2 CPS ≥ 10 were significantly higher in the differentiated histological type (P = 0.015 and P = 0.030, respectively). The I-PD-L1 and I-PD-L2 levels were significantly associated with better CSS (P = 0.037 and P = 0.015, respectively). CD8-high was significantly associated with less lymph node metastasis (P = 0.047), less distant metastasis (P = 0.024), less peritoneal dissemination (P = 0.034), and earlier TNM stage (P = 0.047). The CD8-high group had better prognosis than the CD8-low group (P = 0.018). FoxP3 expression was not associated with any clinicopathological factors or prognosis. We found that patients with PD-L2 CPS ≥ 10 tended to have worse prognosis in the FoxP3/CD8-low group (P = 0.088).
The clinicopathological significance of PD-L1/2 expression may differ depending on the TME status. Immune checkpoint inhibitors may improve the prognosis of SBA patients with low FoxP3/CD8 ratio and PD-L2 expression.
Core Tip: We investigated the clinicopathological significance of programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 (PD-L2) expression in association with the infiltration of FoxP3+ and CD8+ T cells into the tumor microenvironment (TME) to identify PD-L/PD-1 immunotherapy candidates among patients with small bowel adenocarcinoma (SBA). We demonstrated that the status of the TME affects the clinical significance of PD-L1 and PD-L2. PD-L2 may be associated with poor prognosis of SBA patients with a high immune cell infiltration.
- Citation: Hoshimoto A, Tatsuguchi A, Hamakubo R, Nishimoto T, Omori J, Akimoto N, Tanaka S, Fujimori S, Hatori T, Shimizu A, Iwakiri K. Clinical significance of programmed cell death-ligand expression in small bowel adenocarcinoma is determined by the tumor microenvironment. World J Gastroenterol 2023; 29(40): 5566-5581
- URL: https://www.wjgnet.com/1007-9327/full/v29/i40/5566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i40.5566
Small bowel adenocarcinoma (SBA) is an uncommon condition, accounting for less than 3% of all gastrointestinal neoplasms; however, the small intestine accounts for 95% of the surface area of the entire gastrointestinal tract[1]. According to a recent report from the United States, the incidence of small bowel cancer has been increasing, with an annual increase of 1.8% between 2006 and 2015[2]. Although there are no definitive risk factors for SBA, diets containing high volumes of animal fat and protein have been reported to increase the risk, which may be related to an increase in the number of SBA[3]. SBA accounts for 30%–50% of all small bowel malignancies, and limited data exist on its molecular and clinicopathological features[4]. SBA has a high probability of being discovered at an advanced stage owing to a delay in diagnosis. No effective chemotherapy has been established for unresectable SBAs, and the regimens for colorectal cancer are palliatively administered. However, comprehensive genomic analyses have revealed that SBA and colorectal cancer have different genomic profiles, and it is assumed that the molecular pathways leading to carcinogenesis may also be different[3,5,6]. Therefore, it is essential to establish chemotherapeutic regimens based on the specific characteristics of SBA.
The small bowel is the largest organ of the human immune system. Lymphoid tissues in the lamina propria and various immune-related cells are prevalent in the small intestine and contribute to immune surveillance. Programmed cell death-ligand 1 (PD-L1) is the primary PD-1 Ligand that is upregulated in various solid tumors, and plays a pivotal role in modulating the tumor microenvironment (TME) to inhibit cytokine production and the cytolytic activity of PD-1+ tumor-infiltrating CD4+ and CD8+ T cells[7]. Several studies have demonstrated the efficacy of blocking the PD-1/PD-L1 signaling pathway in gastrointestinal cancers with high microsatellite instability (MSI-high)[8-11], and recent studies have demonstrated a significant association between MSI-high and PD-L1 expression in SBA[12,13]. PD-L1 expression has been reported to be associated with gastrointestinal cancer prognosis, despite several conflicting reports. While some studies have reported that PD-L1 expression is associated with favorable prognosis in gastric and colorectal cancers[14-18], others have reported that PD-L1 expression is associated with poorer prognosis in these cancers[19-22]. Considering the role of PD-1/PD-L1 in the TME, PD-L1 expression is predicted to be associated with worse prognosis in cancer patients.
Programmed cell death-ligand 2 (PD-L2) was discovered as a second ligand for PD-1 and has been reported to inhibit T cell proliferation by blocking cell cycle progression, similar to PD-L1[23,24]. PD-L2 suppresses the proliferation and cytokine production of CD4+ T cells through T cell receptors[23]. Its expression is significantly associated with PD-L1 expression in melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, renal cell carcinoma, bladder cancer, gastric cancer, and triple-negative breast cancer[25]. Although the function and expression pattern of PD-L2 are considered to be similar to those of PD-L1, PD-L2 has not received much attention, and its role in modulating tumor immunity remains undetermined.
Anti-PD-L1/PD-1 therapy utilizes tumor-infiltrating lymphocytes (TILs). Therefore, the status of TILs in the TME may influence their efficacy. Although TILs are mainly composed of CD8+ T cells, they are heterogeneous cell populations that also contain regulatory T cells (Tregs), which are believed to inhibit CD8+ T cells[26]. Tregs are an immunosuppressive subset of CD4+ T cells that orchestrate cellular and molecular networks to induce an immunosuppressive environment favorable to tumorigenesis[27]. Tregs are characterized by the expression of the master regulatory transcription factor FoxP3[28]. A high ratio of FoxP3+ to CD8+ T cells is associated with poor clinical outcomes in digestive system cancers[29-31].
This study investigated the clinicopathological significance of PD-L1 and PD-L2 expression in association with the infiltration of FoxP3+ and CD8+ T cells into the TME to identify PD-L/PD-1 immunotherapy candidates among SBA patients.
We obtained 50 duodenal, jejunal, and ileal adenocarcinoma tissue samples from the archives of the Department of Pathology at Nippon Medical School Hospital for immunohistochemical analysis of PD-L1, PD-L2, CD8, FoxP3, and DNA mismatch repair (MMR) protein expression. Samples from patients with predisposing conditions, including Lynch syndrome, familial adenomatous polyposis, celiac disease, and Crohn’s disease, were excluded to focus on sporadic SBA. Furthermore, samples from patients with ampullary adenocarcinoma or metastatic cancer were excluded. Cancer-specific survival (CSS) was defined as the interval from the date of the first surgery until death due to SBA; patients who died due to other causes were excluded. All patients provided informed written consent prior to study enrollment, and the study was approved by the Ethics Committee of Nippon Medical School (approval no. B-2020-164). All staging criteria were defined following the International Union for Cancer TNM classification.
Specimens were fixed in 10% formalin, embedded in paraffin wax, and immersed in 0.5% H2O2–methanol for 10 min to block endogenous peroxidase activity. Subsequently, the sections were microwaved in 0.01 mol/L citrate phosphate buffer (pH = 6.0) or EDTA (pH = 9.0) for antigen retrieval and incubated with 10% normal horse or goat serum for 10 min at 37°C to block nonspecific IgG binding. Thereafter, the sections were incubated for 18 h at 4°C with the primary antibodies listed in Supplementary Table 1. Next, they were treated with their respective biotinylated antibodies, namely anti-mouse IgG or anti-rabbit IgG (1:200; Vector) for 30 min at 25°C, followed by treatment with avidin-biotin-peroxidase complex for 30 min at 25°C. The reaction products were developed by immersing the sections in a 3,3’-diaminobenzidine tetrahydrochloride solution containing 0.03% H2O2.
Each patient was blindly evaluated by two independent observers (A.H. and A.T.). Any disagreements were resolved using a multi-headed microscope. The immunoreactivities of PD-L1 and PD-L2 were determined separately in tumor cells and tumor-infiltrating immune cells, such as lymphocytes and macrophages, throughout the tumor center and invasive margins. Tumor samples were defined as PD-L1 and PD-L2 positive when ≥ 1% of the tumor cells and/or tumor-infiltrating immune cells were immunoreactive with unequivocal intensity. Subsequently, for PD-L1 and PD-L2 expression, a combined positive score (CPS) was calculated by dividing the total number of both tumor cells and immune cells above the positive threshold by the total number of viable tumor cells[32,33]. We set the PD-L1 and PD-L2 CPS cutoffs at ≥ 10%[34].
We assessed CD8+ T cells in ten randomly selected microscopic areas, including the intratumoral and tumor-surrounding stroma, no further than one high-power field from the tumor edge, by light microscopy (400 ×; BX63; Olympus, Tokyo, Japan). FoxP3+ T cells were assessed in the same ten high-power fields. Cell counts were determined using CellSens Dimension software (Olympus, Tokyo, Japan). Immune-related cell densities were graded as low (≤ median cell counts/mm2 tumor area) or high (> median cell counts/mm2 tumor area).
MMR status was defined by immunostaining for MLH1, MSH2, MLH6, and PMS2. Tumors were considered negative when there was a complete absence of nuclear staining of neoplastic cells in the presence of an internal positive control assessed on a whole slide. Tumors with negative staining for one of the MMR proteins were considered deficient MMR (dMMR), and all others were considered proficient MMR (pMMR). Each section was evaluated microscopically (400 ×; BX63; Olympus, Tokyo, Japan).
The immunostaining results for each protein were compared with the clinicopathological factors using the chi-square test or Fisher’s exact test. Association between protein immunostaining was assessed using the chi-square test or Fisher’s exact test, as appropriate. The distribution of CSS was estimated using the Kaplan–Meier method and the log-rank test was used to test for significant differences in CSS. A Cox proportional hazards model was used to assess the effects of tumor variables on CSS. In the multivariate analysis, variables with P < 0.05 in the univariate analysis were included. Statistical significance was set at P < 0.05.
Demographic and baseline clinicopathological characteristics are listed in Table 1. The study included 35 men and 15 women ranging in age from 32 to 84 years (mean age, 65 years; median age, 68 years). At the time of analysis, 18 patients had died. The overall 5-year survival rate was 64%. The median follow-up duration for the entire series was 36 mo (mean, 45.1 mo; range, 5–124 mo). Twenty-three patients with stages III and IV disease received chemotherapy after surgery.
| No. of cases (%) | |
| Age | |
| ≤ 68 | 27 (54.0) |
| > 68 | 23 (46.0) |
| Sex | |
| Female | 15 (30.0) |
| Male | 35 (70.0) |
| Site | |
| Duodenum and jejunum | 45 (90.0) |
| Ileum | 5 (10.0) |
| Histology | |
| WD, MD | 41 (82.0) |
| PD, Muc | 9 (18.0) |
| Depth of invasion | |
| pT1-2 | 14 (28.0) |
| pT3-4 | 36 (72.0) |
| Lymph node metastasis | |
| Absence | 27 (54.0) |
| Presence | 23 (46.0) |
| Distant metastasis | |
| Absence | 37 (74.0) |
| Presence | 13 (26.0) |
| Peritoneal seeding | |
| Absence | 40 (80.0) |
| Presence | 10 (20.0) |
| Chemotherapy | |
| Absence | 27 (54.0) |
| Presence | 23 (46.0) |
| TNM stage | |
| I | 14 (28.0) |
| II | 13 (26.0) |
| III | 10 (20.0) |
| IV | 13 (26.0) |
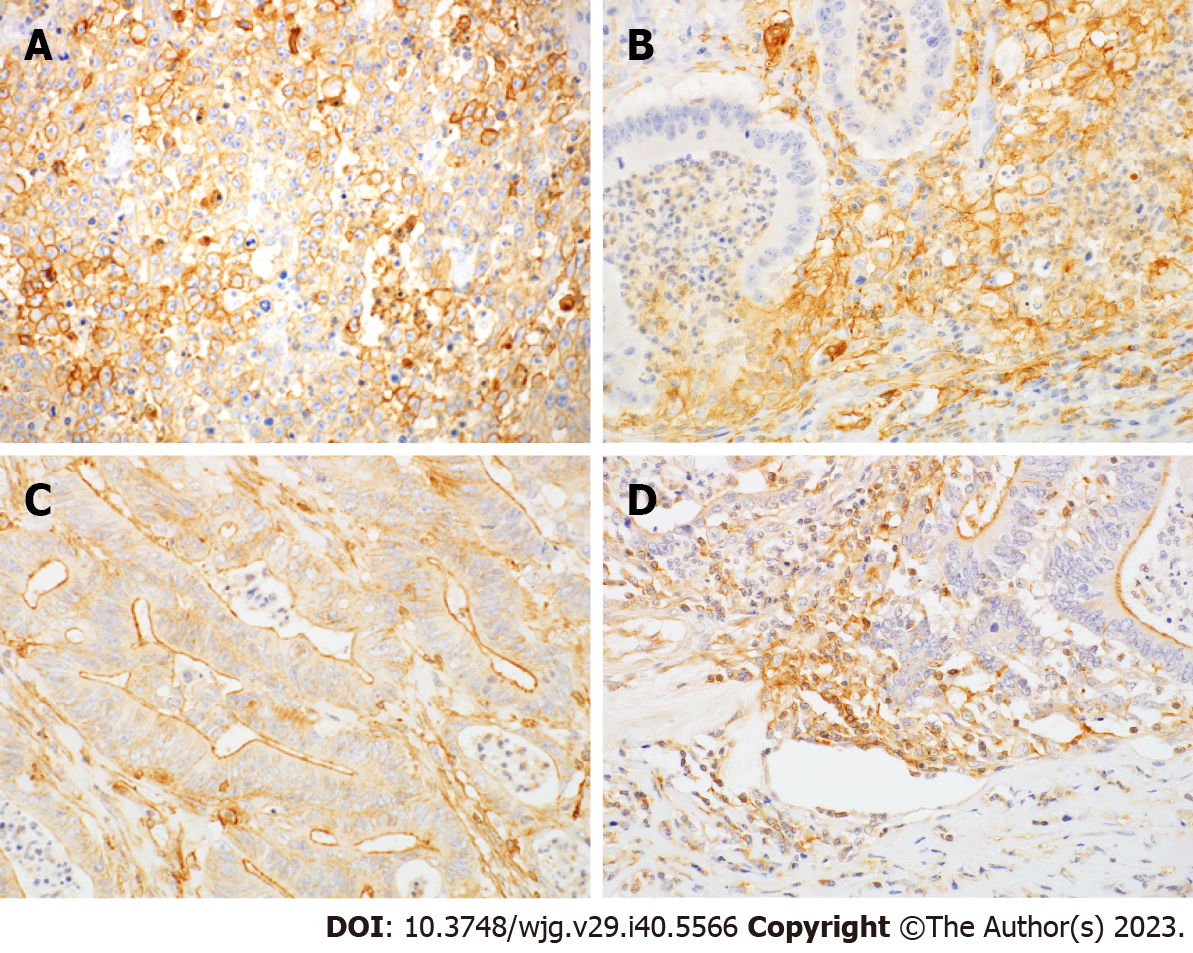
PD-L1 immunoreactivity was observed in both tumor cells (T-PD-L1) and tumor-infiltrating immune cells (I-PD-L1), including lymphocytes and macrophages (Figure 1A and B). PD-L1 immunostaining was membranous and cytoplasmic in tumor cells and membranous in macrophages. The clinicopathological correlation of PD-L1 and PD-L2 expression are summarized in Table 2. T-PD-L1 was positive in 34% and I-PD-L1 was positive in 54% of cases. T-PD-L1 expression was associated with deeper depth of invasion (P = 0.001), but not with lymph node metastasis or distant metastasis. I-PD-L1 expression was associated with deeper depth of invasion (P = 0.024) and less peritoneal metastasis (P = 0.019). Both T-PD-L1 and I-PD-L1 expression were more common in stage II tumors compared to stage I tumors. PD-L2 immunoreactivity was observed in tumor cells (T-PD-L2) and tumor-infiltrating immune cells (I-PD-L2), including lymphocytes and macrophages (Figure 1C and D). The apical membrane showed a predominant PD-L2 staining pattern in tumor cells (Figure 1C). T-PD-L2 was positive in 34% and I-PD-L2 was positive in 42% of cases. T-PD-L2 expression was significantly higher in differentiated histological type (P = 0.015).
| No. | T-PD-L1 | I-PD-L1 | PD-L1 CPS ≥ 10 | T-PD-L2 | I-PD-L2 | PD-L2 CPS ≥ 10 | |||||||
| N (%) | P value | N (%) | P value | N (%) | P value | N (%) | P value | N (%) | P value | N (%) | P value | ||
| Age | |||||||||||||
| ≤ 68 | 27 | 10 (37.0) | NS | 16 (59.3) | NS | 14 (51.9) | NS | 12 (44.4) | NS | 14 (51.9) | NS | 19 (70.4) | 0.027 |
| > 68 | 23 | 7 (30.4) | 11 (47.8) | 11 (47.8) | 5 (21.7) | 7 (30.4) | 9 (39.1) | ||||||
| Sex | |||||||||||||
| Female | 15 | 4 (26.7) | NS | 7 (46.7) | NS | 7 (46.7) | NS | 6 (40.0) | NS | 8 (53.3) | NS | 8 (53.3) | NS |
| Male | 35 | 13 (37.1) | 20 (57.1) | 18 (51.4) | 11 (31.4) | 13 (37.1) | 20 (57.1) | ||||||
| Site | |||||||||||||
| Duodenum and jejunum | 45 | 16 (35.6) | NS | 25 (55.6) | NS | 24 (53.3) | NS | 16 (35.6) | NS | 20 (44.4) | NS | 26 (57.8) | NS |
| Ileum | 5 | 1 (20.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 2 (40.0) | ||||||
| Histology | |||||||||||||
| WD, MD | 41 | 13 (31.7) | NS | 24 (58.5) | NS | 21 (51.2) | NS | 17 (41.5) | 0.015 | 19 (46.3) | NS | 26 (63.4) | 0.030 |
| PD, Muc | 9 | 4 (44.4) | 3 (33.3) | 4 (44.4) | 0 (0.0) | 2 (22.2) | 2 (22.2) | ||||||
| Depth of invasion | |||||||||||||
| pT1-2 | 14 | 0 (0.0) | 0.001 | 4 (28.6) | 0.024 | 2 (14.3) | 0.002 | 5 (35.7) | NS | 6 (42.9) | NS | 6 (42.9) | 0.068 |
| pT3-4 | 36 | 17 (47.2) | 23 (63.9) | 23 (63.9) | 12 (33.3) | 15 (41.7) | 22 (61.1) | ||||||
| Lymph node metastasis | |||||||||||||
| Absence | 27 | 8 (29.6) | NS | 16 (59.3) | NS | 13 (48.1) | NS | 8 (29.6) | NS | 13 (48.1) | NS | 15 (55.6) | NS |
| Presence | 23 | 9 (39.1) | 11 (47.8) | 12 (52.2) | 9 (39.1) | 8 (34.8) | 13 (56.5) | ||||||
| Distant metastasis | |||||||||||||
| Absence | 37 | 12 (32.4) | NS | 22 (59.5) | NS | 20 (54.1) | NS | 13 (35.1) | NS | 18 (48.6) | NS | 23 (62.2) | 0.095 |
| Presence | 13 | 5 (38.5) | 5 (38.5) | 5 (38.5) | 4 (30.8) | 3 (23.1) | 5 (38.5) | ||||||
| Peritoneal seeding | |||||||||||||
| Absence | 40 | 14 (35.0) | NS | 25 (62.5) | 0.019 | 23 (57.5) | 0.034 | 14 (35.0) | NS | 19 (47.5) | NS | 25 (62.5) | NS |
| Presence | 10 | 3 (30.0) | 2 (20.0) | 2 (20.0) | 3 (30.0) | 2 (20.0) | 3 (30.0) | ||||||
| TNM stage | |||||||||||||
| I | 14 | 0 (0.0) | 0.008 | 4 (28.6) | 0.005 | 2 (14.3) | 0.001 | 5 (35.7) | NS | 6 (42.9) | NS | 6 (42.9) | NS |
| II | 13 | 8 (61.5) | 12 (92.3) | 11 (84.6) | 3 (23.1) | 7 (53.8) | 9 (69.2) | ||||||
| III | 10 | 4 (40.0) | 6 (60.0) | 7 (70.0) | 5 (50.0) | 5 (50.0) | 8 (80.0) | ||||||
| IV | 13 | 5 (38.5) | 5 (38.5) | 5 (38.5) | 4 (30.8) | 3 (23.1) | 5 (38.5) | ||||||
| MMR status | |||||||||||||
| Proficient | 43 | 12 (27.9) | 0.037 | 20 (46.5) | 0.009 | 18 (41.9) | 0.005 | 15 (34.9) | NS | 16 (37.2) | NS | 22 (51.2) | NS |
| Deficient | 7 | 5 (71.4) | 7 (100.0) | 7 (100.0) | 2 (28.6) | 5 (71.4) | 6 (85.7) | ||||||
Then, we assessed PD-L1 and PD-L2 expression in a mixture of tumor and immune cells using CPS. Patients with PD-L1 CPS ≥ 10% and PD-L2 CPS ≥ 10% were observed in 50% and 56% of cases, respectively. In patients with PD-L1 CPS ≥ 10% of tumors were associated with deeper depth of invasion (P = 0.002), less peritoneal metastasis (P = 0.034), and more common in stage II compared to stage I (Table 2). Patients with PD-L2 CPS ≥ 10% were significantly correlated with younger age (P = 0.027) and more differentiated histological type (P = 0.030) (Table 2).
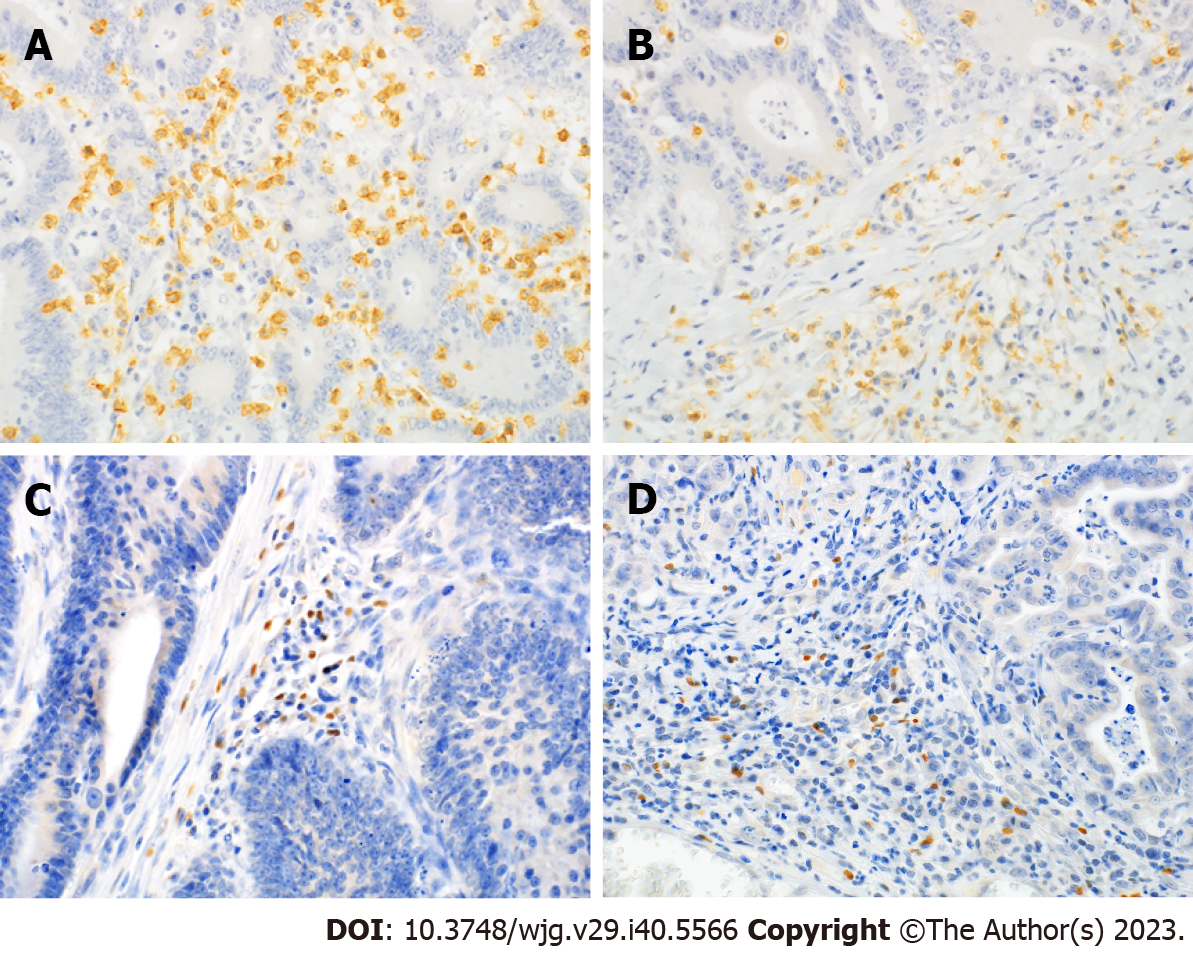
Representative micrographs of CD8 and FoxP3 staining are shown in Figure 2. CD8-high was significantly associated with less lymph node metastasis (P = 0.047), less distant metastasis (P = 0.024), less peritoneal dissemination (P = 0.034), and earlier TNM stage (P = 0.047) (Table 3). FoxP3 immunoreactivities were not associated with any clinicopathological factors, including age, sex, localization, histological type, lymph node metastasis, distant metastasis, peritoneal dissemination, or TNM stage. FoxP3-high tumors had deeper depth of invasion, although the difference was not statistically significant (P = 0.059) (Table 3).
| No. | FoxP3 | CD8 | FoxP3/CD8 | ||||
| N (%) | P value | N (%) | P value | N (%) | P value | ||
| Age | |||||||
| ≤ 68 | 27 | 14 (51.9) | NS | 14 (51.9) | NS | 14 (51.9) | NS |
| > 68 | 23 | 11 (47.8) | 11 (47.8) | 11 (47.8) | |||
| Sex | |||||||
| Female | 15 | 7 (46.7) | NS | 8 (53.3) | NS | 8 (53.3) | NS |
| Male | 35 | 18 (51.4) | 17 (48.6) | 17 (48.6) | |||
| Site | |||||||
| Duodenum and jejunum | 45 | 22 (48.9) | NS | 23 (51.1) | NS | 22 (48.9) | NS |
| Ileum | 5 | 3 (60.0) | 2 (40.0) | 3 (60.0) | |||
| Histology | |||||||
| WD, MD | 41 | 21 (51.2) | NS | 21 (51.2) | NS | 20 (48.8) | NS |
| PD, Muc | 9 | 4 (44.4) | 4 (44.4) | 5 (55.6) | |||
| Depth | |||||||
| pT1-2 | 14 | 4 (28.6) | 0.059 | 7 (50.0) | NS | 4 (28.6) | 0.059 |
| pT3-4 | 36 | 21 (58.3) | 18 (50.0) | 21 (58.3) | |||
| Lymph node metastasis | |||||||
| Absence | 27 | 11 (40.7) | NS | 17 (63.0) | 0.047 | 8 (29.6) | 0.002 |
| Presence | 23 | 14 (60.9) | 8 (34.8) | 17 (73.9) | |||
| Distant metastasis | |||||||
| Absence | 37 | 16 (43.2) | NS | 22 (59.5) | 0.024 | 14 (37.8) | 0.004 |
| Presence | 13 | 9 (69.2) | 3 (23.1) | 11 (84.6) | |||
| Peritoneal seeding | |||||||
| Absence | 40 | 19 (47.5) | NS | 23 (57.5) | 0.034 | 16 (40.0) | 0.005 |
| Presence | 10 | 6 (60.0) | 2 (20.0) | 9 (90.0) | |||
| TNM stage | |||||||
| I | 14 | 4 (28.6) | NS | 7 (50.0) | NS | 4 (28.6) | 0.011 |
| II | 13 | 7 (53.8) | 10 (76.9) | 4 (30.8) | |||
| III | 10 | 5 (50.0) | 5 (50.0) | 6 (60.0) | |||
| IV | 13 | 9 (69.2) | 3 (23.1) | 11 (84.6) | |||
| MMR status | |||||||
| Proficient | 43 | 19 (44.2) | 0.049 | 20 (46.5) | NS | 22 (51.2) | NS |
| Deficient | 7 | 6 (85.7) | 5 (71.4) | 3 (42.9) | |||
Then, we calculated and summed the ratio of FoxP3 to CD8 positive T cell counts in ten high-power fields. FoxP3/CD8-high was significantly associated with lymph node metastasis (P = 0.002), distant metastasis (P = 0.004), peritoneal dissemination (P = 0.005), and TNM stage (P = 0.002) (Table 3).
dMMR was 14% of the cases identified using MLH1, MSH2, MLH6, and PMS2 immunostaining. T-PD-L1, I-PD-L1, and PD-L1 CPS ≥ 10% were significantly higher in the dMMR tumors than in the pMMR tumors. No PD-L2 positivity rate was associated with MMR status (Table 2).
Mutual relationship between PD-L1, PD-L2, CD8, and FoxP3 expression
Mutual relationship between PD-L1, PD-L2, CD8, and FoxP3 expression levels are shown in Table 4. A significant positive relationship was observed between PD-L1 and PD-L2 expression in both tumor and immune cells. A significant positive relationship was observed between CD8-high and T-PD-L1, I-PD-L1, and I-PD-L2 expression. FoxP3-high expression was associated with I-PD-L1 expression, but not with T-PD-L1, T-PD-L2, or I-PD-L2 expression.
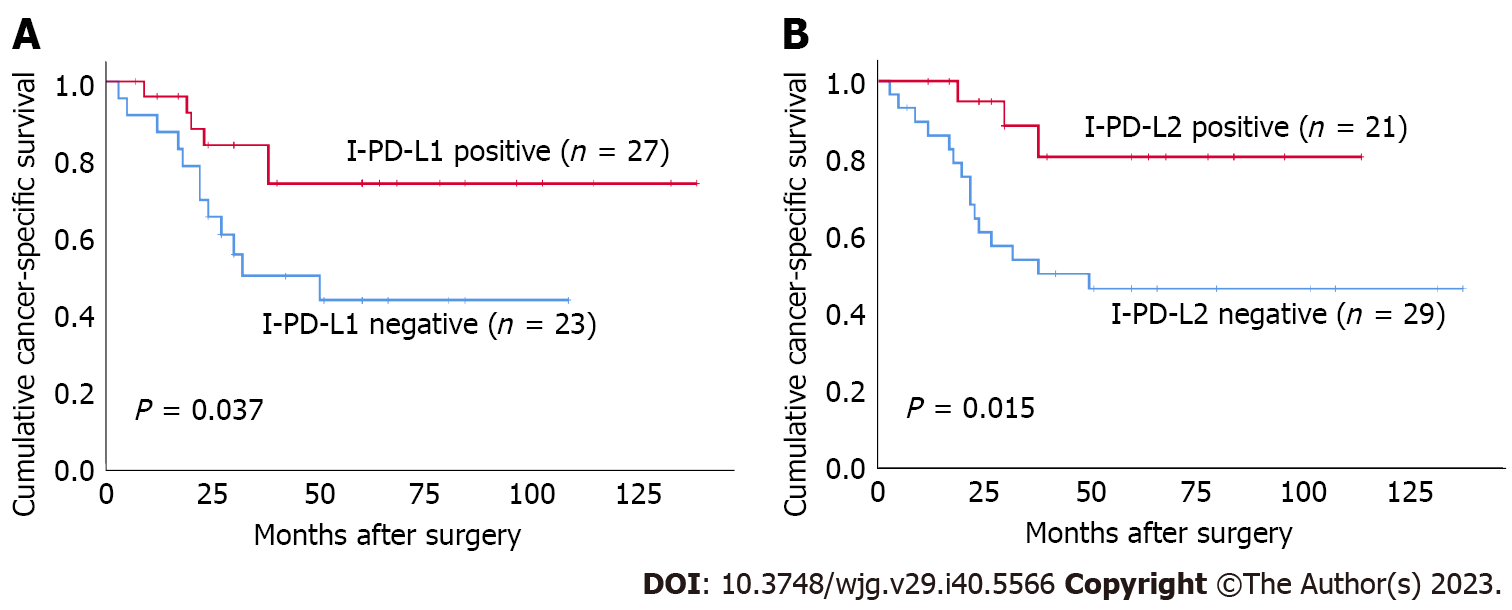
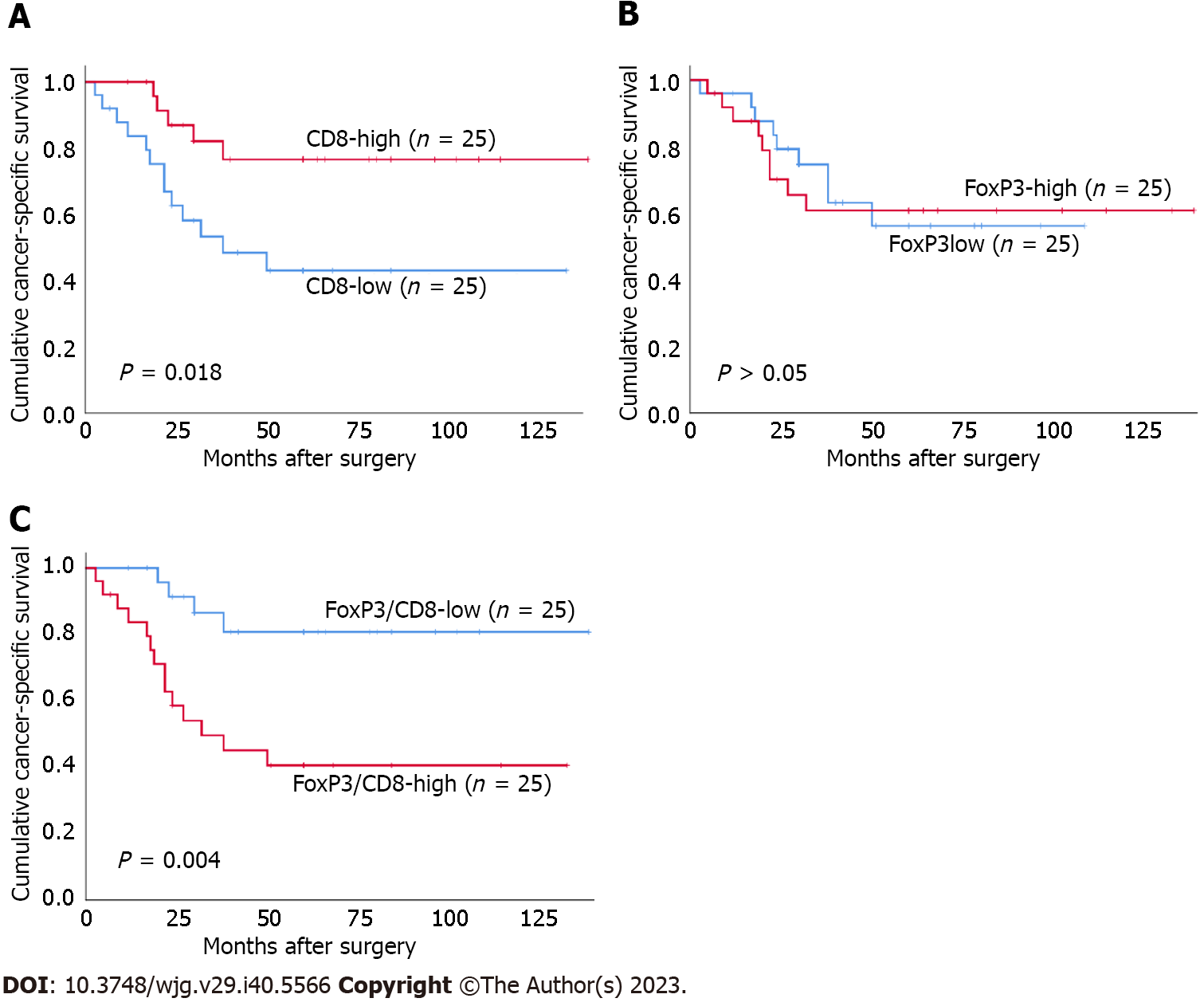
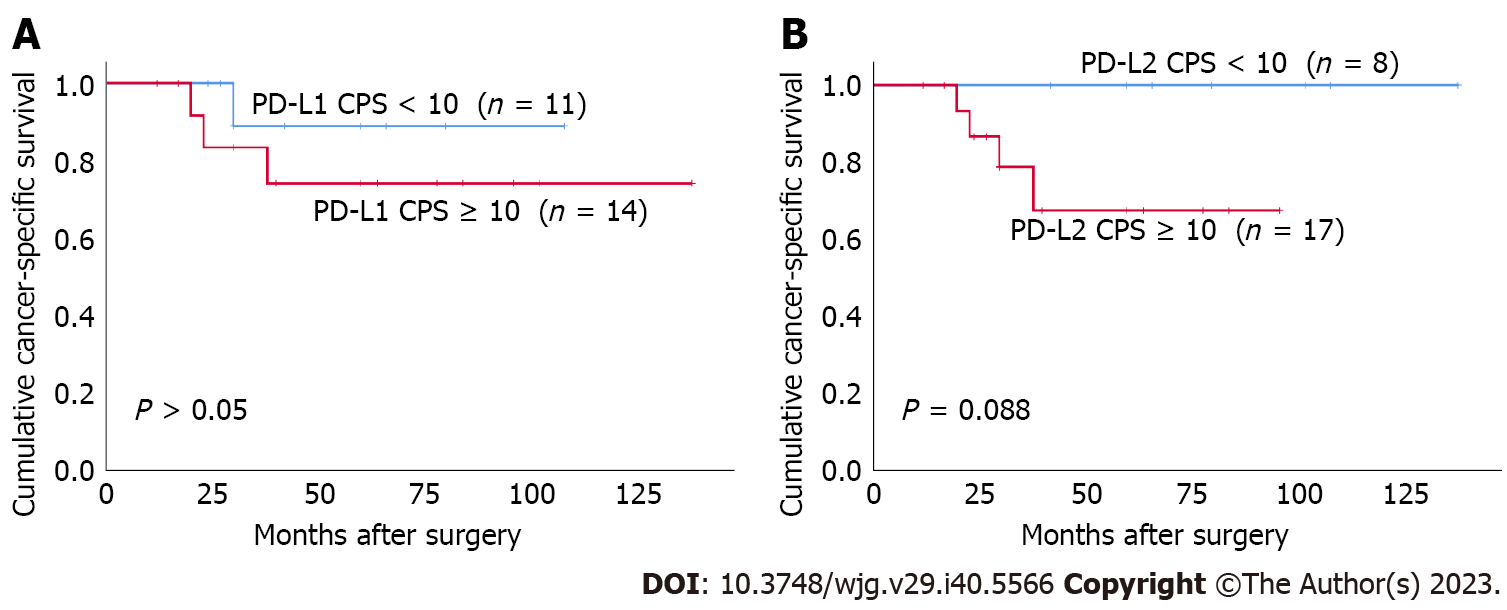
Both I-PD-L1 and I-PD-L2 expression were significantly associated with better CSS (P = 0.037 and P = 0.015, respectively) (Figure 3). There was no significant association between T-PD-L1 expression or PD-L1 CPS ≥ 10% and patients’ survival. There was no significant association between T-PD-L2 expression or PD-L2 CPS ≥ 10% and patients’ survival. Patients in the CD8-high group had better prognosis than those in the CD8-low group (P = 0.018) (Figure 4). There was no significant association between FoxP3+ T cells and patients’ survival. In contrast, the FoxP3/CD8-low group had significantly better prognosis than the FoxP3/CD8-high group (P = 0.004). We performed a survival analysis for PD-L1 and PD-L2 expression stratified by the FoxP3/CD8 ratio, and found that patients with PD-L2 CPS ≥ 10 in the FoxP3/CD8-low group had worse prognosis, although the difference was not significant (P = 0.088) (Figure 5).
In the univariate analysis using the Cox proportional hazards model for CSS, lymph node status and I-PD-L1, I-PD-L2, CD8, and FoxP3/CD8 ratios had significant prognostic value (Table 5). In the multivariate analysis performed by introducing all the above variables into the Cox proportional hazards model, lymph node status and I-PD-L2 expression retained independent prognostic significance.
| Variables | Categories | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Histological type | PD, Muc vs. WD, MD | 2.252 (0.799-6.348) | NS | ||
| Depth | pT3-4 vs. pT1-2 | 39.308 (0.661-2337.048) | NS | ||
| Lymph node metastasis | Positive vs. negative | 42.764 (5.639-324.314) | < 0.001 | 66.320 (7.494-586.923) | < 0.001 |
| T-PD-L1 | Positive vs. negative | 0.733 (0.261-2.059) | NS | ||
| I-PD-L1 | Positive vs. negative | 0.367 (0.137-0.980) | 0.046 | 1.156 (0.231-5.769) | NS |
| PD-L1 CPS ≥ 10 | Positive vs. negative | 0.621 (0.240-1.606) | NS | ||
| T-PD-L2 | Positive vs. negative | 0.702 (0.249-1.981) | NS | ||
| I-PD-L2 | Positive vs. negative | 0.243 (0.070-0.840) | 0.025 | 0.113 (0.020-0.650) | 0.015 |
| PD-L2 CPS ≥ 10 | Positive vs. negative | 0.493 (0.191-1.276) | NS | ||
| FoxP3 | High vs. low | 1.067 (0.423-2.694) | NS | ||
| CD8 | High vs. low | 0.311 (0.111-0.872) | 0.026 | 1.922 (0.164-22.481) | NS |
| FoxP3/CD8 | High vs. low | 4.490 (1.476-13.660) | 0.008 | 1.476 (0.138-15.769) | NS |
In this study, we performed immunostaining for PD-L1 and PD-L2 to investigate their clinicopathological significance in SBA. Since PD-L1 is expressed in both tumor cells and tumor-infiltrating immune cells, we examined the clinicopathological significance of their expression separately to clarify whether there were differences in their roles in tumor progression. Although there were several similarities and correlations, some differences in association with clinicopathological factors were observed. T-PD-L1 expression was associated with deeper depth of invasion, but not with lymph node metastasis, distant metastasis, or prognosis. These results suggest that T-PD-L1 contributes to the local invasion of tumor cells but not to metastasis; consequently, it is not associated with prognosis. I-PD-L1 expression is associated with deeper depth of invasion, less peritoneal metastasis, and favorable prognosis. We speculated that I-PD-L1 expression might be influenced by peritumoral infiltrating T cells, which correlates with favorable prognosis. Indeed, we found a positive relationship between I-PD-L1 expression and the CD8+ T cell count, and a high density of CD8+ TILs was associated with favorable prognosis. The main similarity between T-PD-L1 and I-PD-L1 expression was that both were associated with deeper depth of invasion and were more common in stage II compared to stage I tumors. These results suggest that the clinical significance of PD-L1 does not differ following the cell type in which it is expressed. Based on the above findings, we adopted the CPS to evaluate PD-L1 expression. The CPS was calculated by summing the number of PD-L1–stained cells (tumor cells, lymphocytes, and macrophages) and dividing the result by the total number of viable tumor cells, and multiplying by 100. Based on the observed response rate and response durability, the U.S. Food and Drug Administration granted pembrolizumab accelerated approval for the treatment of recurrent locally advanced, metastatic gastric or gastro-esophageal junction adenocarcinoma that expresses PD-L1 CPS ≥ 1. Thus, the CPS is now becoming a standard method for the assessment of PD-L1 expression. Because the CPS is a scoring system characterized by collectively quantifying positivity for tumor cells and positivity for surrounding immune cells, it may be possible to optimize the presence or absence of PD-L1 expression in carcinomas accompanied by inflammatory or immune cell infiltration. We examined the relationship between PD-L1 expression and clinicopathological factors using the CPS ≥ 10 as a cut-off in this study, resulting in that no significant association between PD-L1 expression and prognosis was found.
It has been demonstrated that PD-L2 is a second ligand for PD-1 and can be expressed by immune, stromal, or tumor cells, mainly through Th2-associated cytokines depending on tumor microenvironmental stimuli[35]. To date, a few studies have reported the significance of PD-L2 expression in gastrointestinal carcinomas[36,37]. To our knowledge, this is the first study to examine the relationship between PD-L2 expression and the clinicopathological characteristics of SBA patients. Since PD-L2 is expressed in both tumor cells and tumor-infiltrating immune cells, we examined the clinicopathological significance of its expression in both cell types, as we did for PD-L1. T-PD-L2 expression was not associated with any clinicopathological factors except for histological type and prognosis. I-PD-L2 expression was significantly associated with better CSS, suggesting that I-PD-L2 expression may be influenced by peritumoral infiltrating T cells that correlate with favorable prognosis. Then, we evaluated the PD-L2 expression using the CPS. PD-L2 CPS ≥ 10 was associated with younger age and was more common in differentiated histological type, but not with prognosis. It has been reported that PD-L2 expression was associated with poor prognosis in gastric and colorectal cancers[36,37]. These findings are intuitively understandable, considering that PD-L2 expression suppresses tumor immunity as well as PD-L1 does. However, several studies have demonstrated that both PD-L2 and PD-L1 expression are favorable prognostic indicators for gastric and colorectal cancer[25,38]. To date, such discrepancies have been explained by differences in scoring methods, cutoff values of immunostaining, heterogeneities of carcinoma, and any bias originating from the inclusion of an insufficient number of cases. To resolve this, we investigated the clinical significance of PD-L1 and PD-L2 expression according to TME status. CD8+ TILs are associated with the tumor immune response and can be used to predict the response to immunotherapy and survival outcomes in CRC[39]. It has been reported a high density of CD8+ TILs is associated with favorable prognosis in CRC[40-42]. We found that CD8-high was negatively associated with lymph node metastasis, distant metastasis, and peritoneal dissemination in SBA patients. We also found that the CD8-high group had better prognosis than the CD8-low group, which is consistent with the findings of previous reports. These results suggest that CD8+ TILs have an anti-tumor effect in SBA as well as in CRC and that CD8+ TILs play an important role in improving patient survival. Then, we analyzed the association between FoxP3+ Tregs and clinicopathological factors. CD4+ Tregs expressing the transcription factor FoxP3 are highly immunosuppressive and play a central role in maintaining self-tolerance and immune homeostasis. FoxP3+ T cells promote tumor progression by suppressing effective anti-tumor immunity by inactivating or reducing the proliferation of cytotoxic CD8+ T cells and CD4+ T effector cells in tumors[27,28]. To the best of our knowledge, this is the first study to examine the relationship between the density of FoxP3+ T cells and the clinicopathological characteristics in SBA patients. We found that FoxP3-high tumors tended to have deeper depth of invasion but were not associated with lymph node metastasis, distant metastasis, and peritoneal dissemination. No association was observed between FoxP3+ T cell density and CSS. This is due to the functional heterogeneity of FoxP3+ T cells, as FoxP3+ non-Tregs are secreted depending on inflammatory cytokines such as TGF-β and IL-12[43]. While some studies have reported that high density of FoxP3+ T cells in the TME is associated with poor prognosis in lung cancer, others have reported that high density of FoxP3+ T cells is associated with favorable prognosis in CRC, bladder cancer, and head and neck cancers[44]. Thus, it has been reported that the amount of FoxP3+ T cells infiltration does not always serve as a poor prognostic indicator in the case of carcinoma accompanied by inflammatory cell infiltration, including CRC[43]. Then, we estimated the ratio of FoxP3+ T cells to CD8+ T cells in 10 HPF selected from the same site in the same section. FoxP3/CD8-high was significantly associated with deeper depth of invasion, lymph node metastasis, distant metastasis, peritoneal dissemination, and TNM stage progression. Patients with higher FoxP3+/CD8+ T cells ratios have poor prognosis for gastrointestinal cancers[29-31]. Similarly, we observed that the prognostic value of this ratio was better than that of FoxP3+ or CD8+ T cells alone, which partly reflects the interactions between anti-tumor CD8+ T cells and immunosuppressive FoxP3+ T cells in tumors[45,46]. These results indicate that assessing both FoxP3+ and CD8+ T cells is more useful for understanding TME status than assessing FoxP3 or CD8 + T cells alone.
Both PD-L1 CPS ≥ 10 and PD-L2 CPS ≥ 10 significantly correlated with increased CD8+ T cells infiltration. Furthermore, I-PD-L2 expression was more frequent in FoxP3/CD8-low tumors. These results may explain why I-PD-L2 expression is associated with favorable outcomes. To determine the effect of PD-L1 and PD-L2 on the prognosis of patients with SBA, excluding the effect of the TME, we divided the patients into FoxP3/CD8-low and FoxP3/CD8-high groups. We investigated the correlation between PD-L1 and PD-L2 expression and the prognosis in the two patient groups. We found that patients with PD-L2 CPS ≥ 10 tended to have poorer prognosis than those with PD-L2 CPS < 10 in the FoxP3/CD8-low group, although the difference was not statistically significant. Furthermore, there were no deaths among patients with a PD-L2 CPS < 10 in the FoxP3/CD8-low group. Generally, cases in which the infiltration of CD8 is dominant over FoxP3 are predicted to have favorable prognosis due to the anti-tumor immune effect of T cells; nevertheless, patients showing poor prognosis are also included within the group. The PD-L2 pathway may contribute to poor prognosis in patients with FoxP3/CD8-low tumors. The results of this study also indicate that PD-L2 may be a better predictive factor for prognosis in these cases than PD-L1.
Recent studies have demonstrated that the tumor mutation burden (TMB) could be a biomarker in patients with cancer treated with immune checkpoint inhibitors (ICIs), in which high TMB is significantly correlated with better survival[47]. Although the efficacy of ICIs in patients with cancer with TMB-high or MSI-high/dMMR has been confirmed, patients with MSI-high have a favorable prognosis, and generally, only a small number of patients require chemotherapy. In fact, none of the patients with dMMR died in our cohort, and only two of seven patients received chemotherapy. Most patients with SBA have microsatellite stable (MSS)/pMMR or TMB-low, and it is necessary to identify useful biomarkers for ICI therapy in patients with MSS/pMMR. Previous studies have shown that TMB-high tumors are immunogenic and that TMB-high tumors are also present in SBA[48]. In small bowel cancer, TMB-high has been reported to be associated with dMMR, CD8-high, and PD-L1 expression, but it has been shown that TMB-high cases are also present in cases of pMMR[49,50]. Our results indicate that PD-L2 positive and FoxP3/CD8-low patients with pMMR may benefit from ICI therapy. Although we did not examine the TMB in this study, it may stratify the outcomes of patients with SBA with PD-L2 positive and FoxP3/CD8-low.
This study has several limitations. First, the sample size is small. In addition, there was a bias in tumor localization. Second, MMR status was determined based solely on the results of immunohistochemical staining for MMR proteins. Third, there were no deaths among the patients with dMMR tumors. Therefore, we could not analyze patients’ prognosis based on the MMR status.
We elucidated a discrepancy in the previously reported clinical significance of PD-L1 expression in SBA. Several studies have reported that PD-L1 expression, which should be involved in tumorigenesis, is associated with favorable prognosis. This contradictory finding may originate from the fact that all patients are equally included in the analysis without considering the TME status and the type of cells in which PD-L1 is expressed. We demonstrated that the clinical significance of PD-L2 may be affected by TME status. Although SBA patients with high immune cell infiltration generally have better prognosis, some of these patients have poorer prognosis. PD-L2 may contribute to poorer prognosis of these patients. ICIs may improve the patients’ prognosis in the FoxP3/CD8-low group through blocking the binding of the PD-1 to PD-L2 and activating locally infiltrated T cells.
According to a recent report from the United States, the incidence of small bowel adenocarcinoma (SBA) has been increasing, with an annual increase of 1.8% between 2006 and 2015. Comprehensive genomic analyses revealed that SBA and colorectal cancer have different genomic profiles, and it is assumed that the molecular pathways leading to carcinogenesis may also be different. Therefore, it is essential to establish chemotherapeutic regimens based on the specific characteristics of SBA.
The clinicopathological significance of programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 (PD-L2) expression in SBA is not yet fully understood. There are several conflicting reports regarding the clinicopathological significance of PD-L1 expression in gastrointestinal cancers. To resolve this discrepancy, we investigated the clinical significance of PD-L1 and PD-L2 expression according to tumor microenvironment (TME) status stratified by the density of FoxP3+ and CD8+ T cells.
In this study, we investigated the clinicopathological significance of PD-L1 and PD-L2 expression in association with the infiltration of FoxP3+ and CD8+ T cells in the TME to identify PD-L/PD-1 immunotherapy candidates among patients with SBA. We elucidated the discrepancy in previously reported clinical significance of PD-L1 expression in SBA.
The immunoreactivities of PD-L1 and PD-L2 were determined separately in tumor cells and tumor-infiltrating immune cells, such as lymphocytes and macrophages, and evaluated using the combined positive score (CPS). To our knowledge, this is the first study to examine the relationship between PD-L2 expression, and the density of FoxP3+ T cells, and the clinicopathological characteristics of patients with SBA.
PD-L1 expression was positive in 34% in tumor cells (T-PD-L1) and 54% in tumor-infiltrating immune cells (I-PD-L1) of the cases examined. T-PD-L2 was positive in 34% and I-PD-L2 was positive in 42% of the cases, respectively. PD-L1 CPS ≥ 10 and PD-L2 CPS ≥ 10 were observed in 50% and 56% of cases, respectively. I-PD-L1 and I-PD-L2 Levels were significantly associated with better prognosis. We speculated that I-PD-L1 expression might be influenced by peritumoral infiltrating T cells, which correlates with favorable prognosis. We found that patients with PD-L2 CPS ≥ 10 tended to have worse prognosis in the FoxP3/CD8-low group. Although SBA patients with high immune cell infiltration, such as those in the FoxP3/CD8-low group, generally have better prognosis, some have poorer prognosis. Therefore, PD-L2 may contribute to the poor prognosis of these patients.
We identified a discrepancy in the previously reported clinical significance of PD-L1 expression in SBA. Several studies have reported that PD-L1 expression, which is involved in tumorigenesis, is associated with favorable prognosis. This contradictory finding may originate from the fact that all patients were equally included in the analysis without considering the TME status and the type of cells in which PD-L1 was expressed. To identify PD-L/PD-1 immunotherapy candidates, not only PD-L1 expression and DNA mismatch repair/microsatellite instability but also PD-L2 expression and the density of tumor-infiltrating lymphocytes, such as FoxP3+ and CD8+ T cells, in the TME should be considered.
In this study, we did not consider tumor mutation burden (TMB). TMB-high has been proposed as a predictive biomarker for the response to immune checkpoint inhibitors based on the assumption that increasing the number of mutant proteins will create antigenic peptides, allowing for enhanced immunogenicity. In the future, we may be able to analyze TMB status in SBA and combine it with PD-L1/2 expression and TME status to generate powerful biomarkers for identifying immunotherapy candidates.
We thank Mrs. Akiko Takeda for her technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qin J, China; Zhe MM, China; Tsoulfas G, Greece S-Editor: Lin C L-Editor: A P-Editor: Lin C
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13300] [Cited by in F6Publishing: 14511] [Article Influence: 2902.2] [Reference Citation Analysis (2)] |
| 2. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Fujimori S, Hamakubo R, Hoshimoto A, Nishimoto T, Omori J, Akimoto N, Tanaka S, Tatsuguchi A, Iwakiri K. Risk factors for small intestinal adenocarcinomas that are common in the proximal small intestine. World J Gastroenterol. 2022;28:5658-5665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 4. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 418] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 5. | Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017;3:1546-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Tatsuguchi A, Yamada T, Ueda K, Furuki H, Hoshimoto A, Nishimoto T, Omori J, Akimoto N, Gudis K, Tanaka S, Fujimori S, Shimizu A, Iwakiri K. Genetic analysis of Japanese patients with small bowel adenocarcinoma using next-generation sequencing. BMC Cancer. 2022;22:723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 7. | Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen SM, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366 (26):2455-2465. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5908] [Article Influence: 492.3] [Reference Citation Analysis (0)] |
| 8. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 1250] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 9. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 838] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 10. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1775] [Cited by in F6Publishing: 1812] [Article Influence: 258.9] [Reference Citation Analysis (0)] |
| 11. | Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist. 2019;24:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | Thota R, Gonzalez RS, Berlin J, Cardin DB, Shi C. Could the PD-1 Pathway Be a Potential Target for Treating Small Intestinal Adenocarcinoma? Am J Clin Pathol. 2017;148:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Noh BJ, Hong SM, Jun SY, Eom DW. Prognostic implications of immune classification in a multicentre cohort of patients with small intestinal adenocarcinoma. Pathology. 2020;52:228-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269-24283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 15. | Seo AN, Kang BW, Kwon OK, Park KB, Lee SS, Chung HY, Yu W, Bae HI, Jeon SW, Kang H, Kim JG. Intratumoural PD-L1 expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Br J Cancer. 2017;117:1753-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233-2242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Wang W, Jing H, Liu J, Bu D, Zhang Y, Zhu T, Lu K, Xu Y, Cheng M, Yao J, Huang S, Wang L. Correlation between schistosomiasis and CD8+ T cell and stromal PD-L1 as well as the different prognostic role of CD8+ T cell and PD-L1 in schistosomal-associated colorectal cancer and non-schistosomal-associated colorectal cancer. World J Surg Oncol. 2021;19:321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 176] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 20. | Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, Tanabe Y, Moriyama J, Fujii T, Matoba S, Kuroyanagi H, Takano T. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108:853-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P, Markowitz AJ, Shike M, Stadler ZK, Vakiani E, Klimstra DS, Shia J. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2021] [Cited by in F6Publishing: 2102] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 24. | Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117-3126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 368] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY, Handa M, Tomassini JE, McClanahan T. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res. 2017;23:3158-3167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 26. | Aristin Revilla S, Kranenburg O, Coffer PJ. Colorectal Cancer-Infiltrating Regulatory T Cells: Functional Heterogeneity, Metabolic Adaptation, and Therapeutic Targeting. Front Immunol. 2022;13:903564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 27. | Saleh R, Elkord E. FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 28. | Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 351] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 29. | Kiryu S, Ito Z, Suka M, Bito T, Kan S, Uchiyama K, Saruta M, Hata T, Takano Y, Fujioka S, Misawa T, Yamauchi T, Yanagisawa H, Sato N, Ohkusa T, Sugiyama H, Koido S. Prognostic value of immune factors in the tumor microenvironment of patients with pancreatic ductal adenocarcinoma. BMC Cancer. 2021;21:1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Zhu Y, Li M, Mu D, Kong L, Zhang J, Zhao F, Li Z, Liu X, Bo C, Yu J. CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget. 2016;7:71455-71465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, Katano M. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59:653-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 615] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 33. | Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J, Lunceford J, Savage MJ, Juco J, Emancipator K. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med. 2019;143:330-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 34. | Wainberg ZA, Fuchs CS, Tabernero J, Shitara K, Muro K, Van Cutsem E, Bang YJ, Chung HC, Yamaguchi K, Varga E, Chen JS, Hochhauser D, Thuss-Patience P, Al-Batran SE, Garrido M, Kher U, Shih CS, Shah S, Bhagia P, Chao J. Efficacy of Pembrolizumab Monotherapy for Advanced Gastric/Gastroesophageal Junction Cancer with Programmed Death Ligand 1 Combined Positive Score ≥10. Clin Cancer Res. 2021;27:1923-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Masugi Y, Nishihara R, Hamada T, Song M, da Silva A, Kosumi K, Gu M, Shi Y, Li W, Liu L, Nevo D, Inamura K, Cao Y, Liao X, Nosho K, Chan AT, Giannakis M, Bass AJ, Hodi FS, Freeman GJ, Rodig SJ, Fuchs CS, Qian ZR, Nowak JA, Ogino S. Tumor PDCD1LG2 (PD-L2) Expression and the Lymphocytic Reaction to Colorectal Cancer. Cancer Immunol Res. 2017;5:1046-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Wang H, Yao H, Li C, Liang L, Zhang Y, Shi H, Zhou C, Chen Y, Fang JY, Xu J. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology. 2017;6:e1327494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Gao Y, Li S, Xu D, Chen S, Cai Y, Jiang W, Zhang X, Sun J, Wang K, Chang B, Wang F, Hong M. Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma. Chin J Cancer. 2017;36:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Pyo JS, Son BK, Chung KH, Oh IH. Clinicopathological significance and prognostic implication of programmed death-1 Ligand 2 expression in colorectal cancer. Int J Biol Markers. 2019;34:276-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Zou Q, Wang X, Ren D, Hu B, Tang G, Zhang Y, Huang M, Pai RK, Buchanan DD, Win AK, Newcomb PA, Grady WM, Yu H, Luo Y. DNA methylation-based signature of CD8+ tumor-infiltrating lymphocytes enables evaluation of immune response and prognosis in colorectal cancer. J Immunother Cancer. 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014; 110(6): 1595-1605. [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 41. | Wang E, Shibutani M, Nagahara H, Fukuoka T, Iseki Y, Okazaki Y, Kashiwagi S, Tanaka H, Maeda K. Prognostic value of the density of tumor-infiltrating lymphocytes in colorectal cancer liver metastases. Oncol Lett. 2021;22:837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1659] [Cited by in F6Publishing: 1864] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 43. | Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 580] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 44. | Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3108] [Cited by in F6Publishing: 3312] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 45. | Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol. 2015;33:101-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2350] [Cited by in F6Publishing: 2217] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 47. | Li DD, Tang YL, Wang X. Challenges and exploration for immunotherapies targeting cold colorectal cancer. World J Gastrointest Oncol. 2023;15:55-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1568] [Cited by in F6Publishing: 1517] [Article Influence: 303.4] [Reference Citation Analysis (0)] |
| 49. | Wirta EV, Szeto S, Hänninen U, Ahtiainen M, Böhm J, Mecklin JP, Aaltonen LA, Seppälä TT. Prognostic Value of Immune Environment Analysis in Small Bowel Adenocarcinomas with Verified Mutational Landscape and Predisposing Conditions. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Pedersen KS, Foster NR, Overman MJ, Boland PM, Kim SS, Arrambide KA, Jaszewski BL, Bekaii-Saab T, Graham RP, Welch J, Wilson RH, McWilliams RR. ZEBRA: A Multicenter Phase II Study of Pembrolizumab in Patients with Advanced Small-Bowel Adenocarcinoma. Clin Cancer Res. 2021;27:3641-3648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |