Copyright
©The Author(s) 2023.
World J Gastroenterol. Oct 21, 2023; 29(39): 5471-5482
Published online Oct 21, 2023. doi: 10.3748/wjg.v29.i39.5471
Published online Oct 21, 2023. doi: 10.3748/wjg.v29.i39.5471
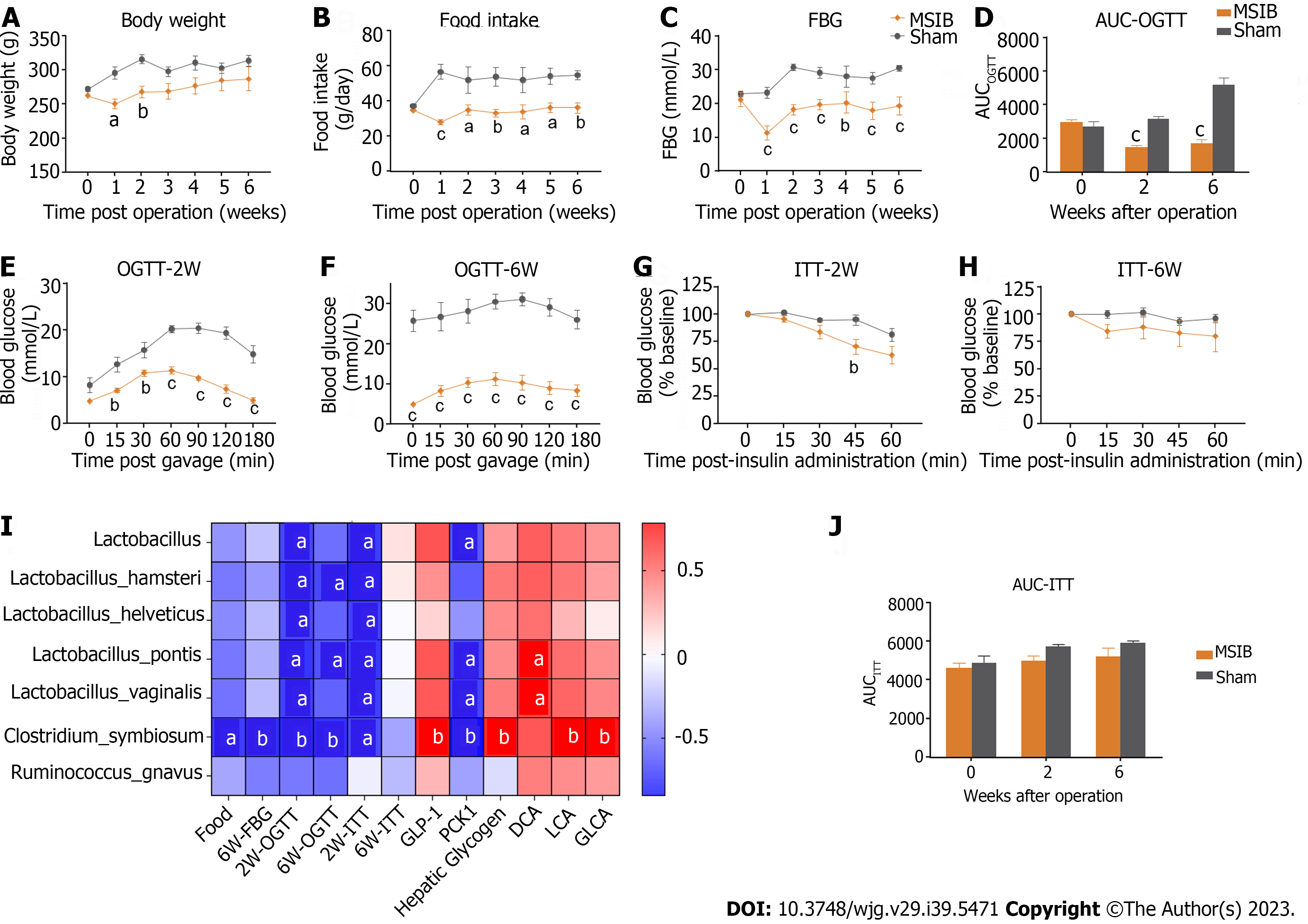
Figure 1 Glucose metabolism improved after mid-small intestine bypass and was significantly correlated with postoperative increased abundance of gut microbiota.
A: Postoperative body weight changes in rats; B: Postoperative food intake in rats; C: Postoperative mean random blood glucose changes in rats; D-F: Glucose tolerance test and area under the curve in rats; G-J: Insulin sensitivity test and area under the curve in rats (G, H, and J); analysis of the correlation between postoperative abundance of rising gut microbiota and glucose metabolism and bile acids (I). Data are expressed as mean ± SEM and statistical significance was determined by two-tailed Student’s test or two-way ANOVA, aP < 0.05, bP < 0.01, cP < 0.001. FBG: Fasting blood glucose; AUC: Area under the curve; OGTT: Oral glucose tolerance test; MSIB: Mid-small intestine bypass; ITT: Insulin tolerance test; PCK1: Phosphoenolpyruvate carboxykinase 1; DCA: Deoxycholic acid; LCA: Lithocholic acid; GLCA: Glycolithocholic acid.
- Citation: Luo X, Tao F, Tan C, Xu CY, Zheng ZH, Pang Q, He XA, Cao JQ, Duan JY. Enhanced glucose homeostasis via Clostridium symbiosum-mediated glucagon-like peptide 1 inhibition of hepatic gluconeogenesis in mid-intestinal bypass surgery. World J Gastroenterol 2023; 29(39): 5471-5482
- URL: https://www.wjgnet.com/1007-9327/full/v29/i39/5471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i39.5471