Copyright
©The Author(s) 2023.
World J Gastroenterol. Aug 28, 2023; 29(32): 4912-4919
Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4912
Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4912
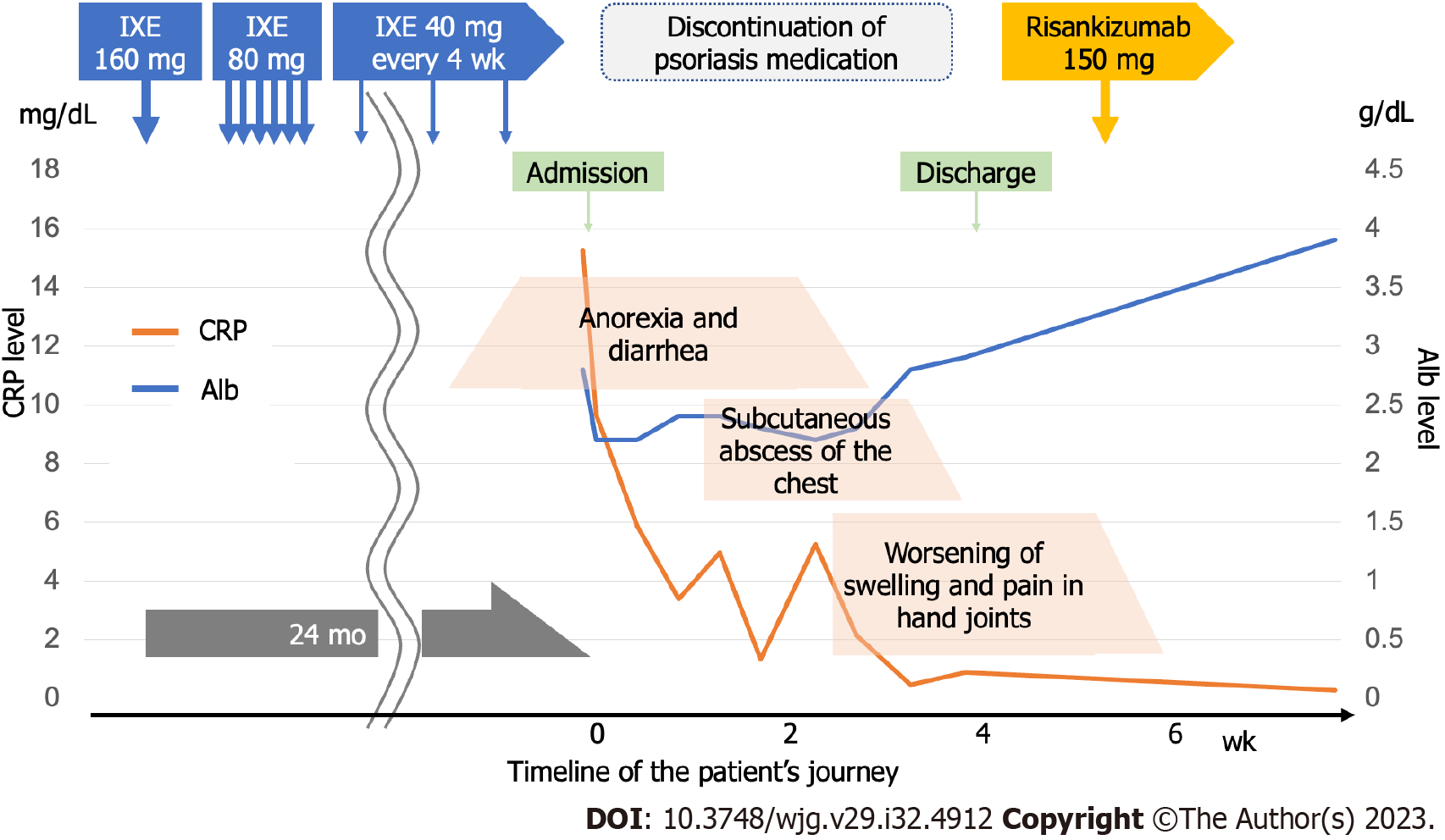
Figure 5 Albumin (Alb) and C-reactive protein (CRP) levels plotted against the timeline of the patients’ journey.
The patient visited our hospital 24 mo after the start of the ixekizumab (IXE) administration. In the graph, CRP is shown on the left vertical axis and Alb on the right vertical axis. Abdominal symptoms gradually improved with IXE withdrawal, but skin and joint symptoms tended to worsen. After 4 wk of hospitalization, the patient was discharged home, as the endoscopy showed that the ulcer had healed and the patient was able to eat adequately. After discharge, risankizumab was introduced to control skin and joint symptoms, and the patients’ condition stabilized. IXE: Ixekizumab; CRP: C-reactive protein; Alb: Albumin.
- Citation: Saito K, Yoza K, Takeda S, Shimoyama Y, Takeuchi K. Drug-induced entero-colitis due to interleukin-17 inhibitor use; capsule endoscopic findings and pathological characteristics: A case report. World J Gastroenterol 2023; 29(32): 4912-4919
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4912