INTRODUCTION
The human organism is colonized by trillions of microbes. The microbiome refers to all the genes of microbes found in various locations in an individual’s body. Instead for microbiota we mean the total of microorganisms quantitatively and qualitatively present. The human host and the microbiota have co-evolved for the benefit of both parties especially the intestinal one[1,2]. In fact, on the one hand, the host provides space, suitable conditions, and food for the growth of the intestinal microbiota and this in turn generally participates in obtaining useful substances and induces resistance to various infections. The relationship between the human gut microbiota and the host is symbiotic, in which both the host and the microorganisms are mutually beneficial. For its part, the host offers a place of growth and nourishment to the symbiotic intestinal bacteria, which in turn favors the function of the host on the one hand by inducing resistance to infections and on the other hand by facilitating the absorption of digested food[3]. It appears, therefore, that eukaryotic hosts and symbiotic bacteria have “co-evolved” with mutual interactions based on nutritional benefits enjoyed by both parties. When this balance is disturbed (dysbiosis) for various reasons, such as repeated and inappropriate use of antibiotics or alcohol abuse, pathological conditions may arise, such as mild chronic intestinal inflammation or metabolic disorders[4]. The interactions of (symbiotic) microbes with each other is of particular interest. A negative correlation has been observed between members of the phylum Bacteroidota, (such as that of the Prevotellaceae spp.) in the intestine, which may reflect alternative “metabolic specializations”[2,5]. Thus, the gut microbiota is of particular importance for the maintenance of human health. In fact, various biological mechanisms are microbiota-dependent because they cannot be performed autonomously and are useful for health homeostasis. In general, the concept of a “superorganism” refers to the bidirectional and therefore beneficial activity between the host organism and the gut microbiota. The microbiota, in addition, has both a protective and trophic role influencing, as we have mentioned, several homeostatic processes of the host organism, e.g., tissue trophism, immune balance, metabolic activity, neuro-endocrine function, etc.[6,7]. Intestinal microbiota represents the most populous community of the entire organism. In fact, the stomach has about 103-104 bacteria, the duodenum 105-106 and the terminal ileum 108-109 bacteria (per gram of tissue). However, the most populated is the large intestinal one representing around 1012-14 bacteria per gram of tissue[2,6]. The colon is the part of the gastrointestinal tract where a complex set of microorganisms develops from the moment of birth. The microbiota of the large intestine is denser and more diverse than the human microbiota of the small intestine. Intestine are enriched in the phyla of Bacillota and Actinomycetota, while Bacteroidota and Lachnospiracae are more abundant in colonic samples[8]. It is estimated that about 400-500 different genera of microorganisms constitute the intestinal microbiota, while the 90% of them species are predominantly anaerobic. Most of them belong to two genera, Bacteroidota and Bacillota. The remaining bacterial populations belong to Pseudomonadota, Actinomycetota, Fusobacteria and Verrucomicrobia[9]. An estimated 70% of these microbial communities are bacteria that cannot be cultured with conventional microbiological techniques. It has been observed that the microbial populations of the intestinal mucosa differ from those found in the intestinal lumen. The large intestine hosts the most numerous microbial cells which vary in species from individual to individual and are organized in localized microbial communities along its path. These microorganisms appear not to follow stable topological patterns along the intestine as they are influenced by the local oxygen concentration. Thus, facultative aerobic communities reside near the intestinal walls, which are sites of high oxygen concentration, and anaerobes prefer the intestinal lumen, where oxygen concentration is lower[10]. Although there is great diversity in the microbes that colonize the gut, it has been found that the gut microbiota of most individuals can be classified into three main microbial groups or “enterotypes” depending on the predominance of genera: Bacteroides (enterotype 1); Prevotella (enterotype 2) or Ruminococcus (enterotype 3). More recent data, however, show that the division into three enterotypes is a simplification and that there are many intermediate states in the gut. The prevalence of each enterotype is mainly determined by dietary factors[2]. However, it appears that everyone has a fixed bacterial strain, even though the composition may vary. One of the main functions of the intestinal microbiota is to protect the intestine from pathogenic microorganisms, it also contributes to the development of a healthy immune system, regulates intestinal motility and participates in metabolism. More specifically, the gut microbiota promotes the regulation of the immune system through: (1) Stimulation of the immune response against potential pathogens; and (2) Suppression of the immune response against food and symbiotic antigens[11]. It is estimated that 80% of antibody production occurs locally in the gut, mainly supplying immunoglobulin A. In addition, they participate in host metabolism, drugs metabolism, maintenance of mucosal structural integrity, bile salts metabolism, plant fibers, mucus, and fatty acid catabolism[12]. In addition to bacteria that have already been studied quite a lot, recently it has been hypothesized that yeasts are involved in starch metabolism. Understanding the relationship between yeasts and the immune system is characterized as difficult and there are few data on their action. In some special cases, they can also act therapeutically[13]. Thus, there is an amphidromic relationship between microbes and hosts (Figure 1).
Figure 1 The cross-talking axis host/gut microbiota: The gastrointestinal microbiota plays an important role in host physiology, metabolism, and nutrition.
An organism with a regular maintenance of the physiological homeostasis leads to eubiosis of the gut microbiota and vice versa. Conversely, an altered physiological homeostasis leads to gut microbiota dysbiosis and vice versa. An alteration in the gut microbial community is linked to several disturbances gut conditions, including cancer, obesity, and a variety of gut disorders. The contribution of beneficial components of the gut microbiota to host physiology, metabolism, and immune function has become the focus of scientific research and will undoubtedly lead to new therapeutic approaches.
Finally, the probiotics bacteria are the “good” bacteria that are found in gut microbiota but also are added in specific dietary or ferment foods. Probiotics aid the organism by strengthening our immunity and help with gastrointestinal health, especially in conditions such as the irritable bowel syndrome (IBS)[14]. In the 1989, Fuller listed some beneficial effects and therapeutic applications of probiotic bacteria. We find probiotics in dairy products, such as yogurt and aged cheeses, but also in other foods such as pickled vegetables, etc. The consumption of probiotics promotes the growth of desirable microorganisms, overcoming potentially harmful bacteria and strengthening the body’s defences. Several scientific articles commenting on these results, refer to studies using cultures such as of Lactobacillus acidophilus and Bifidobacterium, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus[14,15]. Consuming probiotic products can cause a plethora of positives effects on the human body. Probiotics, thanks to their antimicrobial properties action can fight intestinal and other diseases, aid in various infections (such as those from coronavirus disease 2019 and other), reduce levels serum cholesterol, stimulate the immune system, reduce allergy symptoms, aid with lactose intolerance, can prevent hypercholesterolemia and osteoporosis and other[15].
MAIN FACTORS AFFECTING THE INTESTINAL MICROBIOTA
Inheritance
The mechanisms of host-bacteria interactions have not been fully described. Little is known about the relationship between the host genotype and its gut bacteria. However, there is strong evidence that it affects intestinal populations. Simple genetic mutations can lead to changes in the composition of microorganisms in the gut[16]. Further investigations are needed to delineate the mechanisms by which this occurs. There are few studies comparing the gut microbiota between family members. It has been observed that monozygotic and dizygotic twins had a greater similarity in gut microbiota between monozygotic than dizygotic siblings, indicating the importance of genetic background. Furthermore, it was observed in an animal model that the similarities in the gut microbiota of the same mouse strain were greater than in mice of a different species even with a common environmental effect. The role of parental genetic influence on the colonization of microorganisms in the intestine has not been elucidated.
Geographical location
The diversity of the gut microbiota in children living in rural areas is greater than that of children in developed countries. It was noted in a study among Caucasians and Asians (Chinese) in the United States and Hong Kong that there were qualitative differences and quantitative in the intestinal microbiota[17-19]. It was found that children from a country in West Africa (Burkina Faso) that had a high presence of Bacteroidota with a greater presence of the genus Xylanibacter and Prevotella (which allow the hydrolysis of xylan and cellulose) with a reduced presence of Bacillota. Indeed, the higher presence of short-chain fatty acids (SCFAs) was noted. Therefore, the intestinal microbiota can help the host to optimize its energy intake from dietary fibers according to nutrition and needs, thus also protecting against infective and inflammatory processes[20].
Intrauterine period and delivery
There is a growing body of data suggesting that the human gut microbiota begins before infant birth. Meconium (the baby’s first stool) contains bacteria, where Bacillota phyla predominate, the bacteria through the placenta and circulatory system enter the intestinal lumen of the foetal intestine. After human birth, the gut is colonized by many microbial strains, and everyone has their own gut microbiota which changes further throughout their life[2,6]. How the baby is born is very important as it affects the variety of microorganisms which will be installed. During normal delivery the new-born receives microorganisms from the mother’s vagina or intestinal tract, whereas in caesarean section the new-born is exposed to those from the hospital environment. The gut microbiota in these newborns can be disturbed for months to years. Newborns born with normal delivery develop a microbiota that reflects the vaginal microbiota, while those born with caesarean section develop a skin microbiota resulting in delayed microbial colonization by Bacteroides, and Bifidobacterium spp.[16,21].
Diet
Many are observed in the first months of a person’s life changes in the gut microbiota, while the stability and diversity of microbiota genes increase after the first three years of life[21]. Breastfeeding or consuming breastmilk substitutes in infancy has significant effects on intestinal microbiota of the new-born and in the development of the immune system[22]. In animal models, the two different ways of feeding babies develop different populations of microorganisms in the gut which lead to differences in the immune system. Immunity is affected up to the first five years of life[23]. In humans there are few studies on the different administration of milk to newborns. Undoubtedly, however, the intestinal microbiota of the child influences the development of the immune system and its metabolism, just as in adult life[24]. Breastfed infants are exposed to the gut microbiota offered by milk, which contains more than 700 species of bacteria. Breast milk contains many protective factors that breast milk does not contain. In addition, changes in the infant’s gut microbiota appear to predispose to diseases and his later life[2,16]. Subsequently adult dietary habits are one of these most important environmental factors shaping the growth of microorganisms in the human gut. Short or long-term consumption of animal or vegetable products changes the structure of the intestinal microbiota. The short-term diet based on animal foods increases the microorganisms of genera such as Bacteroides, Alistipes, Bilophila and decreases the levels of Roseburia, Eubacterium rectale, Ruminococusbromii belonging to the Bacillota phyla (populations that mainly metabolize polysaccharides of plant origin)[25]. Instead, it has been noted that long-term consumption of fruit and vegetables by the elderly is associated with an increase in populations of the genus Prevotella. It was noticed that a change of nutrition style (i.e., sugar-rich foods, a shift from a low-fat vegetable polysaccharide to a high-fat vegetable polysaccharide, and so) modifies the microbiota, qualitatively and quantitatively, through a change of the microhabitat in 24 h and so on changes in metabolic pathways also occur. Although the gut microbiota responds to short-term changes in diet, long-term nutrient changes appear to determine the type of qualitative and quantitative microorganism population also for other such as the oral ones[26,27]. There are differences in the presence of gut bacteria in the two diets, reflecting differences in metabolism. of carbohydrates and proteins. In both cases, the colonization of fungi, viruses and yeasts is observed[25]. It has been observed that the consumption of foods of plant origin is also associated with a beneficial set in the intestinal microbiota. A high correlation has been noted between a plant-based diet and increased levels of SCFAs and some dietary fibres that degrade Bacillota phyla. Indeed, the characteristics of the Mediterranean-type diet, i.e., increased intake of cereals, fruit, vegetables, and legumes offer many benefits for the human intestine[28]. An observational study found that dietary fiber from beans, fruits, and vegetables was associated with an abundance of and Actinomycetota phyla and Clostridium spp. But also, the consumption of sour milk, many dairy products, or the consumption of fermented foods (such as Greek yogurt, kefir, and others) affect the intestinal microbiota and change its structure. The use of substances of abuse (such as alcohol, cocaine and other) or chemical xenobiotics can cause in human health is associated with quantitative and qualitative changes in the intestinal microbiota (dysbiosis)[29,30].
Lifestyle
Modern lifestyles can influence the intestinal microbiota, sedentary life contributes to obesity and combined with a positive energy balance and the consumption of foods of animal origin rich in saturated fat leads to the rearrangement of populations at the intestinal level. Although studies show conflicting results, an increase in Bacillota and a decrease in Bacteroidota have been observed in individuals consuming foods of animal origin[6]. Frequent and moderate exercise influences the intestinal defence as well as on the genes of intestinal microorganisms. In a study on the effect of exercise on the intestinal microbiota of 22 athletes, the beneficial influence of exercise on the diversity of intestinal microorganisms is underlined and the combination with diet is claimed to affect the gut microbiota structure[31,32]. Smoking also affects the composition of the gut microbiota, increasing populations of the Bacteroides-Prevotella genera. Stress affects gut motility, which can change the structure of microbial populations, which has even been blamed for the development of inflammatory bowel disease (IBD)[33].
Use of antibiotics
Antibiotics target pathogenic microorganisms, but they also have a negative effect on intestinal symbionts. Unfortunately, they affect the intestinal microbiota especially if they are broad-spectrum antibiotics that are used for all diseases[34]. Gut microbiota diversity is reduced, many strains are lost, and their re-emergence is gradual and long-term, which is a major concern of experts in the field of intestinal health. It was observed that most of the bacteria affecting antibiotics response mainly belong to the Bacillota and Pseudomonadota phyla[35,36].
THE ROLE OF THE HOST/INTESTINAL MICROBIOTA CROSS-TALKING AXIS
Nutritional and gut-influenced energy conservation by metabolic processes (metabolome)
Several studies have shown that the intestinal mucosa needs to be colonized by microorganisms to take on its integral structure. For example, mice raised in a sterile environment developed fewer blood vessels in their intestinal villi. Sterile growth also showed that there is defective growth in gut-associated lymphoid tissue and antibody production. Also, in the context of the sterile environment, fewer Peyer’s patches develop, there are fewer cells in the dermis, and fewer plasma cells in the germinal centres of the mesenteric lymph nodes than in the development data in a nonsterile environment[37]. The intestinal microbiota could be considered a “metabolic organ” performing multiple functions needed for maintaining the health status of the host organism orchestrating an amphidromous communication with it. The catabolism of a number of both endogenous and exogenous indigestible molecules (i.e., cholesterol, fibers, bile acids, excreted mucus, etc.), is one of the most important activities of the intestinal microbiota accounting for 10% of the host’s energy requirement every day[38,39]. So, the gut microorganisms (especially bacteria and commensal fungi) produce SCFAs to gain energy but also give trophic substances and energy to the host organism. Finally, certain bacterial species can synthesize vitamins such as B12, folic acid, thiamin, biotin, vitamin K, amino acids and more[40]. As proof, Bacteroides thetaiotaomicron can metabolize polysaccharides that reach the large intestine as is in Figure 2. Indeed, it has many enzymes such as glycoside hydrolases and polysaccharide lyases that break down pectins, arabinose, etc.
Figure 2 The metabolic activity by the colonic microbiota.
The intestinal microbiota finds an environment rich in polysaccharides which are not digested by stomach enzymes. Fermentation of polysaccharides by intestinal bacteria leads to the production of acetate, butyrate, and propionate, which are used as a carbon source by intestinal mucosal cells. The initial fermentation of the carbohydrate that escaped digestion in the small intestine is followed by the utilization and cross-distribution of metabolites by various members of the microbiota, and then the synthesis of short-chain fatty acids (butyrate, propionate, acetate). Proteolytic fermentation differs from saccharolytic fermentation because it releases many potentially toxic nitrogen and sulfur metabolites, such as ammonia, amines, nitrates, nitrites, and hydrogen sulfide[32,40].
Archaea, such as Methanobrevibacter smithii, establish beneficial relationships with other bacteria to eliminate the H2 by-products, thus facilitating the yield of ATP[41,42]. The gut microbiota benefits the host in many ways contributing to the conservation of energy from several common non-digestible polysaccharides through enzymes such as glycoside hydrolases and other non-encoded enzymes in the human genome[43]. Studies of mice with a germ-free gut microbiota revealed that the gut microbiota improves the regulation of fat accumulation and obesity mainly due to an increase in energy production from food. These (germ-free) mice are protected against obesity and metabolic syndrome[44]. It was noted that restoring an eubiotic intestinal microbiota in these animals leads to an increase in insulin resistance and fasting blood glucose, but also of the level of liver triglycerides and body fat amount.
The intestinal microbiota improves the absorption of monosaccharides, which in turn leads to an increased lipogenesis with consequent accumulation of triglycerides both in the liver and in the fat tissue. With food utilization, obese mice have been reported to conserve energy from food more efficiently than lean-wild-type mice[45]. Distal gut microbiota’s composition of obese mice revealed that the relative increase of Bacteroidota and Bacillota resulted in a modified metabolic potential of the gut microbiota, giving it a greater ability to use energy from the diet. Interestingly, this obesity trait of his was transmissible through faecal transplants from obese mice as opposed to lean germ-free mice. Obese mice also possess multiple methanogenic archaea which can increase the efficiency of bacterial fermentation through H2 removal[46]. It was observed that, after gut co-colonization, both Bacteroides thetaiotaomicron and Methanobrevibacter smithii boost up the efficiency and specificity of bacterial fermentation by removing bacterial polysaccharides, increasing adiposity compared to mice colonized with only one of the two organisms[47,48]. Further studies have highlighted further correlations regarding the possibility of increased energy saving through induced microbiota nutrition or genetically induced obesity microbiota[49]. For that, age and diet are important factors not only for the composition of the gut microbiota, but also for energy saving possibilities. In addition, the link between gut microbiota and liver diseases has been studied mainly in obesity and non-alcoholic steatohepatitis/nonalcoholic fatty liver disease patients, and in liver failure conditions (e.g., cirrhosis, hepatic encephalopathy, infections, hepatocellular carcinoma), total parenteral nutrition-associated liver disease, cholangitis primary sclerosing, primary biliary cirrhosis[50]. It has been noted that dysbiosis of the intestinal microbiota leads to intestinal motility disorders (stasis, with bacterial proliferation). These conditions lead to increased intestinal permeability, impaired immune response with an excessive increase in tolerance in bacterial translocation by the intestinal microbiota with subsequent liver injury[50,51].
Immunological actions and resistance to colonization by pathogens
The host’s organism meets both the pathogenic microbes of the environment and the microbes of the intestinal microbiota. Previous studies of the immune system have focused on the mechanisms by which this system can defend itself against pathogenic microbes. In addition, the microorganisms of the intestinal microbiota produce antimicrobial substances such as bacteriocins and hydrogen peroxide which inhibit the growth of others with pathogenic behaviour[51]. The immune system has evolved in such a way that it can accommodate symbiotic bacterial communities of increasing complexity while retaining the ability to fight pathogenic bacteria. The microbiota regulates the development and function of the innate and acquired immune systems[11]. Even in healthy conditions of the host, there is a continuous stimulation of the immune system by the intestinal microbiota. This condition leads to a basal state of “low physiological inflammation” representing an effective first line of defence against pathogenic microbes. Furthermore, both resident and pathogenic microbes compete for available sites and nutrients, so the microbiota exerts a protective role metabolizing those nutrients that are necessary for the pathogens survival and producing molecules that inhibit their growth[2,52]. In fact, it has been shown that the introduction of certain molecules produced by Bacteroides thetaiotamicron and Eubacterium rectale can induce the production of specific mucosal glycans. These can be metabolized by these 2 bacterial species only but not by pathogens, thus preventing their proliferation. Hence, diet appears to have a pivotal role in microbial composition modifications[53]. The immune system works by learning, i.e., at the beginning of life it has the necessary components (cells, internal cellular mediators, etc.) but does not have data available from the environment, which it acquires in the first years of life through contact with other people and the natural environment. If these early childhood data are inappropriate, the regulatory mechanisms of the immune system could fail. As a result, the immune system attacks not only pathogenic microorganisms but also harmless targets such as pollen, house dust and food antigens, leading to the onset of allergic diseases[54]. The microorganisms together with digestive enzymes, the mucus layer, intestinal peristalsis, and the epithelial barrier with “tight” connections (tight junctions) constitutes the non-immune component of the body’s immune response. The functions of the intestinal microbiota in terms of defence of the organism are on the one hand to influence in a decisive way the arise of the gut immunity (for which reference was made to the trophic role) and on the other to prevent possible invasion of pathogens through a direct effect on them and/or through the “activation” of the host’s immune mechanism[55,56]. As far as natural immunity is concerned, it has the ability, by recognizing characteristic pattern molecules [pathogen associated molecular patterns (PAMPs)] on microorganisms, to separate potentially pathogenic microbes from “unwanted” antigens. More specifically, the cells of the natural immunity using proline rich proteins (PRPs) receptors (pattern recognition receptors) detect PAMPs[57]. PRPs also participate in the release of cytokines and the activation of acquired immunity. However, there are many types of PRP, and of the most important are the Toll-like receptors (TLRs) found in dendritic cells, neutrophils, macrophages, and intestinal mucosal epithelial cells. The most known PAMPs recognized by PRP receptors are bacterial carbohydrates (e.g., mannose, glucides of lipopolysaccharide), bacterial peptides (e.g., flagellin), peptidoglycans and lipoteichoic acid of Gram+, fungal lipoproteins, glycans, viral genomes. Since these molecules are also found in symbiotic microorganisms, they are called microbe-associated molecular patterns (MAMPs)[58]. Thus, MAMPs seem to be able to modify the expression of TLRs in natural immunity cells. Thus, the recognition of MAMPs triggers the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, which results in the production of cytokines, the activation of other auxiliary and necessary molecules on the antigen-presenting cells, which ultimately results in the activation of T-cells, i.e., acquired immunity[59]. However, microorganisms can modulate the natural immunity changing the quality and the amount of mucus from the intestinal mucoid cells. Mucus forms a natural barrier to stop an infection directly adhering to them, collects the bacterial by-products, and protect epithelial cells from secretions thanks to its low pH rich and richness in lytic enzymes. These events activate body’s defences[60]. As far as the acquired immunity in the gastrointestinal system is concerned, it is “based” in the gut associated lymphoid tissue which is made up of the Peyer’s Patches and the mesenteric lymph nodes. The spleens of germ-free mice were found to contain increasingly smaller germinal centres in the lymph nodes and reduced numbers of memory CD T4 cells in the intestinal epithelium, that the production of cytokines belongs to a Th2-type of immune response, and that these animals have a reduced ability to secrete germicidal agents[61]. Recolonization of these muscles with muscle-specific bacteria can reverse some of these perturbations, as experimentally demonstrated by the restoration of systemic T-cell deficiency and Th1/Th2 imbalance of sterile muscle microbes after single colonization of the muscle gut with the bacterium Bacteroides fragilis. Such studies collectively highlight the importance of the gut microbiota for the normal development of the peripheral immune system in immunocompetent hosts[62].
Influence of the host’s health condition
According to clinical studies that have been done in recent years, they show a correlation of the change in the composition of the gut microbiota with several serious pathological conditions such as: autoimmune diseases, type 2 diabetes (T2D), weight gain and obesity, IBD, asthma and chronic sinusitis, mental health disorders, dermatological problems, Alzheimer’s disease, poor immune system health, gastroesophageal reflux disease, constipation or diarrhea, cancerous conditions and other[63].
INTESTINAL MICROBIOTA ROLE IN DISEASES
IBS and IBD
Functional bowel disorders such as IBS are defined solely by symptom-based diagnostic criteria. IBS is characterized by abdominal pain or discomfort and changes in bowel habits. Although the aetiology is multifactorial, recent studies to understand the pathophysiology of IBS have revealed that changes in the normal gut microbiota may play a role in IBS-associated low-grade intestinal inflammation[2,40]. Indeed, intestinal microbial dysbiosis is involved in the pathogenesis of IBS by facilitating the adhesion of the pathogenic microorganism to the intestinal wall and several studies revealed that. Indeed, there is a clear separation between the gastrointestinal microbiota of patients with IBS and that of healthy controls[64]. That is, IBS is characterized by an increase in the genera Dorea, Clostridium (as Bacillota phylum) and Ruminococcus with an important loss in the population of the genera Bifidobacterium and Faecalibacterium. According to a trial on IBS in young patients who were divided into subgroups with different bacterial fingerprints, an increase of Bacillota phylum compared to Bacteroidota phylum exists, differing from healthy patients[65,66]. Finally, another study on a paediatric population with IBS showed a variation of Bacillota and Pseudomonadota phyla amount, with many of the genera Dorea and Ruminococcus, and Haemophilus parainfluenzae species. The Bacteroides genus was found to be smaller in paediatric patients with IBS than in healthy controls. Typically, these studies could ultimately lead to the design of targeted therapies[67].
Idiopathic IBDs are immune-driven chronic diseases and represented by ulcerative colitis and Crohn’s disease. Today they are an adverse immune response to endogenous symbiotic gut microorganisms with or without the involvement of the autoimmunity process. Studies have shown a change in the composition of the gut microbiota in IBD[68]. Furthermore, there is a qualitative reduction in it, i.e., on the bio-diversity of the population with a typical reduction of Bacillota phylum strains (such as Bifidobacteria, spp., Faecalibacterium prausnitzii, and Lactobacillaceae families) while an increase in the microorganisms that are attached to the mucus is observed. Bacillota strains are the main producers of SCFAs, such as butyric acid, which has immunomodulatory properties[69]. However, it has not been clarified whether the disturbance in the microbiota is the cause of the disease or is the result of it. The normal, non-inflamed gut contains many immune cells which are in such a state of activation that there is no complete immune response to the microbes of the normal microbiota and food antigens. This is due to the activation of potent mechanisms of immune regulation, such as the stimulation of regulatory T cells expressing the transcription factor FoxP3 to suppress inflammation[66]. But, under environmental stimuli (e.g., certain infections) the activation of the intestinal immune system starts on a large scale but is subsequently suppressed. In IBD patients this suppression of this immune response may not be adequately regulated. The eubiosis of the intestinal microbiota is under the control of the host through immune and epithelial responses, diet, drugs use (especially antibiotics), genetics, and more[2]. In turn, the microbiota, has significant effects on host epithelial and immune function thanks to its structural components and metabolism, that can become permanent by epigenetic effects. From childbirth, when the human microbiota is established, these host effects may influence the risk of developing IBD later in life[63]. It is worth noting here that in most studies the incidence of IBD increases particularly in the second to fourth decades of life, while some studies even report a second peak in the sixth and seventh decades. Specifically, therefore, components of the microbes can promote or protect against disease. The community microbes in patients with ulcerative colitis and Crohn’s disease have been shown to be different from unaffected individuals, a state of dysbiosis: The presence of disease-causing microorganisms (such as those form Pseudomonadota phyla and adherent Escherichia coli) and to which directed immune response and/or loss of microorganisms that inhibit inflammation (e.g., such as Faecalibacterium prausnitzii). But many changes and inflammation results in changes in the microbial community. Also, antibiotics (such as nitroimidazoles, quinolones) and certain diets change the gut microbiota and may improve Crohn’s disease symptoms. In fact, the use of antibiotics really has a place in the treatment of Crohn’s disease[2,16,70].
Immune dysregulation (allergies and autoimmunity)
Effectively stimulated immunity of both local and systemic mucosa is required for a mature gut microbiota. If this does not happen, a dysregulation can occur which can lead to allergic manifestations or an asthmatic phenotype from early life. It has been noted that developing countries have a lower incidence of allergic diseases such as asthma than countries developed. Thus the “hygiene hypothesis” was developed, which according to this lack of exposure to pathogenic bacteria or products of non-pathogenic bacteria can cause this condition by a negative effect on the development of the immune system[71]. Subsequently, the “microbiota hypothesis” was formulated, in which it is proposed that changes in diet, as well as increased use of antibiotics in developed societies, lead to a less diverse gut microbiota in its microbial components. This “immature” gut microbiota, as it has been defined, alters the development of the immune system interrupting the proper evolution of events that allow the development of immune tolerance and thus increasing the risk of developing allergic hypersensitivity[72]. More specifically, levels of Bifidobacterium and Enterococci appeared to be related to allergic symptoms in the first months of life. An increased Bacteroidota/Bifidobacterium ratio was reported during the second year of life in children who developed symptoms of atopy children who eventually developed allergies were less frequently colonized with Lactobacillaceae phyla, Bifidobacterium and Clostridiodies difficile strains during the 2nd mo of life[73]. Autoimmune diseases (such as rheumatoid arthritis and other) can be sharing a common pathogenesis, an immune-mediated attack on the body’s own organs. Autoimmune diseases usually show a variety of characteristics and symptoms. Concerning these characteristics, the most common may include, for example, an increase in epithelial (such as intestinal) and vessel permeability, mitochondrial dysfunction, progressive inflammation and chronic infections, imbalance of the hypothalamic-pituitary-adrenal (HPA) axis, and microbiota dysbiosis. Symptoms, on the other hand, can manifest as chronic fatigue, allergic phenomena, poor cognitive function, mood and mental disorders and pain, skin rashes, gastrointestinal disorders, and so[74,75]. An example of the genetically predisposed autoimmune diseases of the small intestine is celiac disease, an age-independent condition. The symptoms occur after the ingestion of the toxic epitopes of gluten (i.e., the proteins present in wheat, rye, barley and, less so, in oats). In celiac disease there is an increase in intraepithelial lymphocytes and an atrophy of the intestinal villi. The autoimmune mechanism is due to the presence of various autoantigens, where the most important is tissue transglutaminase that is also an important diagnostic marker. Furthermore, it is often associated with other autoimmune diseases, such as insulin dependent diabetes mellitus (IDDM), or type 1 diabetes (T1D), thyroiditis, etc. This suggests that these diseases share common pathogenetic pathways[76-79]. Finally, the intestinal microbiota seems to play a fundamental role in the development and course of celiac disease. There is an unfavourable qualitative and quantitative composition of the intestinal microbiota characterized by a greater presence of the genus Bacteroides and Escherichia coli and a minor presence of Bifidobacterium spp. (e.g., B. longum compared to healthy controls). Furthermore, this condition does not seem to change even after a gluten-free diet[80,81]. Finally, it has been noted that children born by caesarean section have a higher risk to develop the disease[82,83].
Diabetes mellitus
The exact role of the gut microbiota in the pathogenesis of T1D remains unknown. Data from experimental models support the idea that some bacterial families may act protectively against divalent metal transporter 1 (DMT1). Studies have shown that the use of antibiotics in experimental animals can prevent the occurrence of type 1[84]. But also, the administration of probiotics with bacterial strains in experimental animals prevented the onset or delayed the progression of the disease[85]. In another study, it was found that accidentally infecting mice with a spore forming bacterium resulted in a reduction in the incidence of DMT1[86]. Similarly, incubation of mycobacterium and streptococci in laboratory animals protected them from developing diabetes. Studies of the gut microbiota in mice that developed DMT1 and mice that did not develop diabetes reported that, at the onset of the disease, the two groups differed in the concentrations and type of microbes[87]. Stool samples from animals (mice) that developed diabetes contained higher concentrations of so-called probiotic bacteria, such as from Lactobacillaceae phyla and Bifidobacterium spp., in contrast to mice that did not develop diabetes which showed higher concentrations of Bacteroides, Eubacterium and Ruminococcus. It is worth mentioning that the Lactobacillus johnsonii strain prevents the development of diabetes when administered to mice[88,89]. Because of the possible relationship between the microbiota and IBD, most of the research around the gut microbiota has been done in patients with IBD, whereas studies in patients with type 1 diabetes mellitus (T1DM) are limited. In addition to increased intestinal permeability, patients with T1DM show an increase in inflammatory cells in the gut and decreased numbers of CD4, CD25, and T-cells, which are the master regulator of the immune system. Another study showed that individuals who developed T1DM had higher concentrations of Bacteroidota and lower concentrations of Bacillota compared to healthy controls. In addition, individuals who developed DMT1 were colonized with a lower number of bacteria compared to healthy controls[90,91].
The manifestation of T2DM is due to the combination of reduced insulin secretion from the β-cells of the pancreas and increased insulin resistance, as well as the disruption of incretin secretion from the gastrointestinal system. Several studies associated the microbiota with the development of T2DM, which is particularly characterized by a decrease in the concentrations of the phylum Bacillota (such as the genus Roseburia from the Lachnospiraceae family and the Enterococcus faecalis from genus Enterococcus, and other). Changes in the number of Bifidobacteria, Lactobacillaceae phylum, Clostridioides genus, as well as the Bacillota/Bacteroidota ratio have also been observed in the intestinal microbiota of children with T1D[91-93]. Obesity and T2D share the presence of low-grade inflammation that occurs in tissues involved in the regulation of metabolism, such as the liver, adipose tissue, and muscle. Inflammation is characterized by an increase in cytokines, interleukin (IL)-6, IL-1 and tumor necrosis factor-alpha with the result being insulin resistance. There are several studies in which the gut microbiota has been associated with the presence of obesity, insulin resistance and T2D, such as, and that probiotic treatment affects the metabolic control of patients with T2D. The gut microbiota contributes to the development of T2D[94]. Both studies showed that people with T2D had reduced concentrations of Clostridiales bacteria (Roseburia spp. and Enterococcus faecalis)[95]. T2D, independent of obesity, may also affect the structural composition of the microbiota. T2D may have a high presence of Gram-negative intestinal bacteria, such as Bacteroidota. In fact, in diabetic mice a reduction in Bacteroides/Prevotella spp. has been linked to an improvement in metabolic endotoxemia and lowering of laboratory tests of inflammation[96]. Changes in the number of Bifidobacterium, Lactobacillaceae phylum, Clostridium as well as the ratio Bacillota/Bacteroidota have also been observed in the intestinal microbiota of children with T1D[97]. Similar changes in the composition of the intestinal microbiota have been reported in patients with T2D. A lot of different theories have been proposed about the biomechanisms to explain the effect of the intestinal microbiota on insulin resistance and T2D, the main ones being metabolic inflammation, modification of incretin secretion and the production of hydroxybutyric acid. Lipopolysaccharides are endotoxins that are usually found in the outer membrane of Gram-negative bacteria and cause so-called metabolic inflammation, which is characterized by the release of pro-inflammatory factors[98]. The role of lipopolysaccharides in the pathogenesis of metabolic diseases was demonstrated by a study in mice fed a normal diet, in which the infusion of lipopolysaccharides caused insulin resistance in the liver, glucose intolerance, as well as an increase in adipose tissue. In addition, lipopolysaccharides may can lead to the expression of NF-κB and stimulate the activity of mitogen-activated protein kinase metabolic pathways in adipocytes. A study in fed leptin-deficient mice physiologically showed that increased intestinal permeability to lipopolysaccharide resulted in a change in the proportion of Gram-negative bacteria in the intestinal lumen, which was associated with the presence of insulin resistance. The modification of the intestinal microbiota by administering probiotic treatment to obese mice acted favourably on the intestinal barrier, reducing lipopolysaccharide-induced metabolic inflammation[99]. It has been found that increasing concentrations of Bifidobacterium modifies the inflammatory response in obese mice by increasing the production of glucagon like peptides (GLPs), while reducing intestinal permeability. The increase in Bifidobacterium concentrations induced by probiotic treatment is probably associated with an increase in the levels of gut-secreted peptides GLP-1 and YY, which exert a favourable effect, reducing insulin resistance and improving β-cell function. In addition, probiotic treatment caused an increase in GLP-2 levels in the colon, improved intestinal barrier function, and ultimately reduced plasma lipopolysaccharide levels[100]. It was found that people with T2D had an increase in the number of various opportunistic gut pathogens and a decrease in the concentrations of hydroxybutyric acid-producing bacteria. Hydroxybutyric acid is the main source of energy for maintaining the function of the cells of the digestive system. In the large intestine, hydroxybutyric acid is mainly produced by the bacteria Clostridium coccoides and Eubacterium rectale. However, changes in gut bacteria were found in colon cancer patients and in elderly subjects, suggesting that hydroxybutyric acid-producing bacteria could potentially have a protective role in the functioning of the gut microbiota[101,102].
Obesity and atherosclerosis
In obese individuals the accumulation and thus the increase of energy is related to the transfer of hydrogen between certain bacterial taxa. In fact, this effect has been noted both from the hydrogen-producing Prevotellaceae and from the methanogenic archaea that use hydrogen[103]. In obese individuals this increase and accumulation of energy due to the relative abundance of Gammaproteobacteria and the less presence of Clostridium genus. Additionally, obese individuals harbour clusters of H2-producing bacteria, primarily members of the family Prevotellaceae and some groups of Bacillota. These H2-producing bacteria coexist in the gastrointestinal tracts of obese individuals with relatively large numbers of H2-oxidizing methanogens belonging to archaea. Methanogens account up to 10% of all anaerobes in the colon[104,105]. Plant polysaccharides and dietary fibers are fermented by intestinal bacteria to produce SCFAs. An increase in methanogenic oxidation of H2 facilitates fermentation, which produces more SCFAs[106]. It can also be utilized directly by hydrotrophic methanogenic agents, while propionate, butyrate and lactate can be fermented with acetate and H2, where the latter is utilized by hydrotrophic methanogenic bacteria. As a consequence, the increase in methane oxidation should increase the conversion of plant polysaccharides into SCFAs, especially the acetate. SCFAs produced by fermentative bacteria are absorbed through the human intestinal epithelium, whereas H2 is an energy exchange factor within bacterial communities[107].
Atherosclerotic vascular disease is caused by environmental and genetic factors such as food and associated microorganisms. We currently know three circulating phospholipid-related molecules considered promoters of atherosclerosis: Trimethylamine N-oxide (TMAO), choline and betaine that could be used as biomarkers to predict cardiovascular disease risk[108]. These three phospholipid-related molecules were identified by analysis of plasma metabolites from 50 patients with atherosclerotic disease using liquid chromatography/mass spectrometry compared to 50 healthy samples. It has been noted that used apoE-deficient mice as a model of atherosclerosis and showed that plasma TMAO levels in apoE-deficient mice correlated positively with the area of aortic damage. The activity level of hepatic flavin monooxygenases, which convert trimethylamine (TMA) to TMAO, correlated positively with plasma TMAO levels in both mice and humans[109,110]. In the apoE-deficient mice an antibiotic treatment modify plasma TMAO level and atherosclerosis size, suggesting that gut microbiota significantly influence the development of atherosclerosis in apoE-deficient mice reducing it. This was demonstrated by adding 1% choline to the diets of apoE-deficient mice, as it increased the formation of foam cells as well as the expression of the scavenger receptors CD36 and SRA1 on macrophages, which is normally prevented by the administration of broad-spectrum antibiotics. A new pathway linking dietary lipid intake, gut microbiota and atherosclerosis has been found. Dietary L-carnitine is metabolized to TMA by gut microbiota and further converted to TMAO in the liver, which accelerates atherosclerosis in mice[111]. 16S rRNA sequencing of cecum bacteria in mice fed a diet supplemented with L-carnitine showed that the family Prevotellaceae were increased and positively correlated with plasma TMA level[112]. Furthermore, circulating TMAO in individuals with non-selective diet seems to be much higher than in those with a vegetables-based diet. Faecal microbiota analysis and plasma TMAO levels have been correlated in individuals with Prevotella enterotype in respect of those with Bacteroides enterotype, showing that these are higher in the first. L-carnitine use in individuals with different diet habits showed that a varied diet led to the production of more TMAO than in vegans or vegetarians. This confirm that both diet and lifestyle can change both the gut microbiota composition and its ability to metabolize TMA and TMAO from dietary L-carnitine[113]. Based on metagenomics, the genus Collinsella in faecal microbiota seems to be increased in patients with symptomatic atherosclerosis, while the genera Eubacterium and Roseburia is abundant in healthy subjects. Furthermore, in patients with atherosclerosis, metabolomics of faecal microbiota suggests an increase of the expression of genes coding for peptidoglycan synthesis, and a fall of hydrogenase levels. In addition, serum carotenoids, especially β-carotene, were decreased suggesting that symptomatic patients with atherosclerosis could have changes in the gut microbiota and a basic inflammatory state[113,114].
Neurological and psychiatric disturbance
As for the brain, everyone knows that it sends messages throughout the body. The intestine seems to respond. One of the most interesting effects of probiotics on the body focuses on the presence of the gut-brain axis (GBA). The GBA is the connection and the two-way communication between the gut [enteric nervous system (ENS)] and the central nervous system (CNS). This axis is a complex two-way pathway essential for metabolism homeostasis, the influence it has on emotions, mood and in general higher cognitive functions. It is a complex system in which it participates the CNS, the autonomic nervous system, the brain, the spinal cord, and the HPA axis[2,115]. The GBA axis indicates the bidirectional relationship and interdependence between CNS and ENS. It is important to understand this interaction and how a healthy intestinal microbiota can affect the body’s nervous system and vice versa. Through a multitude of mechanisms, hundreds of substances and neurotransmitters, the relationship between the two complex systems is in constant change which can have both positive and negative effects. Stress is a quite common factor that can affect mood, psychology and, apparently, the body’s intestinal microbiota[116,117]. When the organism exposed to a stressful social situation even for a period of only two hours, the microbiota undergoes significant changes, a significant change in its profile and change in the ratio of the main races of bacteria. Additionally, the brain, under the right conditions, can influence composition and functionality of the intestinal microbiota. It may alter intestinal permeability thereby allowing bacterial antigens to enter the epithelium and stimulate a mucosal immune response. Acute stress can increase colonic permeability, leading to overproduction of interferon-γ[116]. Finally, dysbiosis, i.e., alterations caused in the gut due to stress, facilitate the expression of infectious bacteria. An example is the secretion of norepinephrine during surgery which causes expression of Pseudomonas aeruginosa, which can lead to intestinal sepsis. In addition, norepinephrine can stimulate the proliferation of enteric pathogens and increase the infectious properties of Campylobacter jejuni[117-119]. Finally, it can favour the overgrowth of non-pathogenic Escherichia coli isolates, as well as pathogenic Escherichia coli type 0157:H7[3,116]. In continuation of the above, it seems that probiotics have the mechanisms to deal with the complications of stress. Cortisol is a substance produced when the organism is in a state of stress and experiences situations related to anxiety and depression. It was found that probiotics led to a decrease in its release of this substance. In addition, the metabolic products of probiotics, SCFAs, offer beneficial actions. The ENS becomes a recipient of bacterial metabolites. SCFAs such as butyric, acetic, and propionic acids are the main metabolic products of bacterial metabolism[120]. In addition to their essential presence for the multitude of beneficial actions they offer, they can stimulate the sympathetic nervous system, release serotonin in the mucosa and positively affect memory and the learning process[116]. The above-mentioned negative effects of unpleasant psychological states indicate this strong dependence and relationship between CNS and ENS. It is worth focusing on the reverse course, that is, how the health of the body’s microbiota can, through probiotic bacteria, give the appropriate signals to the brain to influence possible diseases. Studies show that balance in the microbiota is related to our emotions, as well as how our brains process information from our senses, such as sights, sounds, tastes. Scientists suspect that gut microbiota disorders may play a role in autism spectrum disorders, depression, anxiety, and chronic pain[121]. The GBA is a communication system between the gut and the brain via neural, hormonal, and immune circuits, offering the gut microbiota and its metabolites a potential pathway to access the brain. This communication system is bidirectional and allows the brain to regulate gastrointestinal functions such as peristaltic movements and mucus production as well as immune functions[122]. Considerable progress has been made in the past decade in understanding the ways in which the gut microbiota is linked to the brain. Stress conditions affects the gut microbiota composition and that two-way communication between gut microbiota and the CNS influences the host’s response to stress. Stress has been shown to affect the integrity of the intestinal epithelium and alter peristalsis, secretions, and mucus production, thereby altering the gut microbiota environment and causing changes in microbial composition and/or metabolism[2,123]. Finally, regarding autism, it has been found that children with autism typically have a higher abundance of Pseudomonadota, Bacteroidota and a lower abundance of Bacillota and Bifidobacteria than healthy children. In fact, many classes of bacteria that make up Bacillota and Clostridia spp., have been found to appear in higher percentages in autistic children with a history of gastrointestinal problems, while at the same time and despite the overall higher abundance of Bacteroidota, and lower Prevotella were observed. Therefore, in addition to quantifying the relative increase or decrease of the populations of some phyla, it has been deemed necessary to determine the populations of specific intestinal symbiotic organisms to understand the significance of some physiological changes in the gut and/or brain[124].
Colorectal cancer
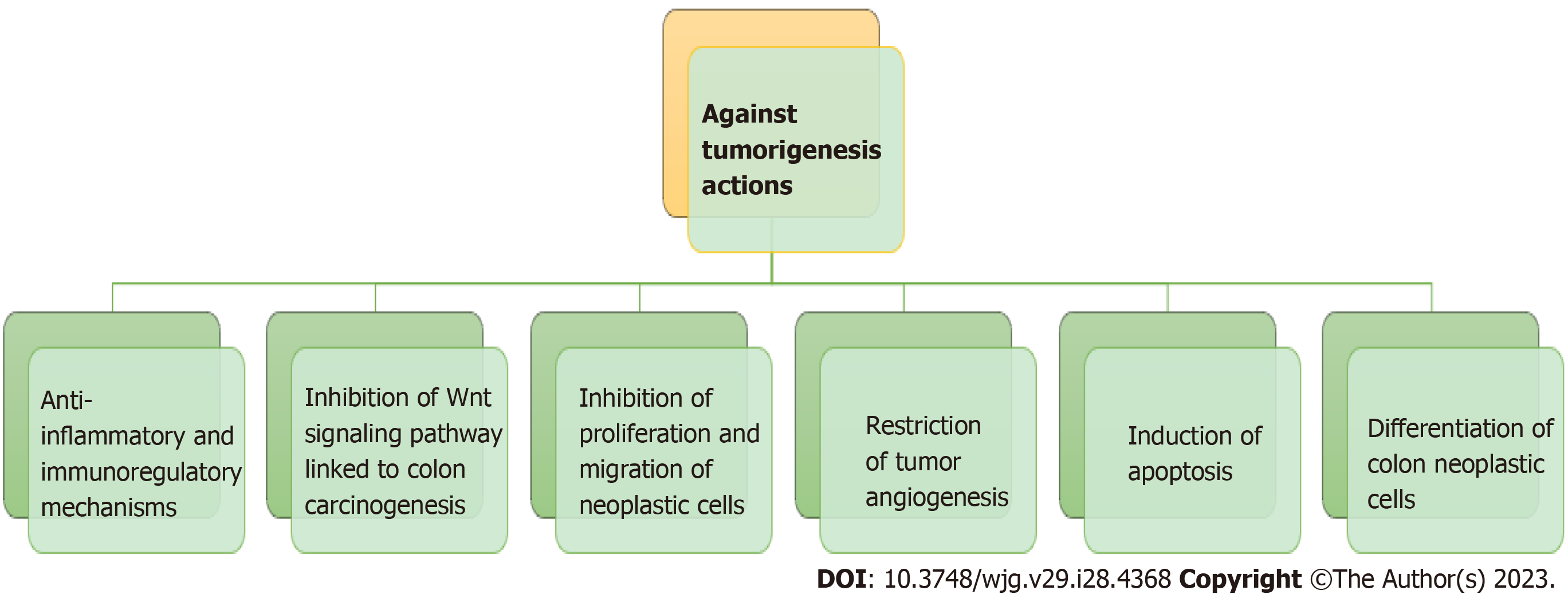
Colonic metabolism may be protective against carcinogenesis under eubiotic conditions[125] (Figure 3). Several environmental and individual factors associated with the development of colorectal cancer, and the interactions between them. It is estimated that 20% of human cancers are related to conditions of chronic inflammation and/or persistent infections. As for example Helicobacter pylori infection is often associated with ulcer and gastric cancer, hepatitis B virus and hepatitis C virus can facilitate hepatocellular carcinoma, IBDs can develop colorectal cancer[126]. The composition of the gut microbiota according to studies may contribute to the mechanism of carcinogenesis either through diet or through its anti-inflammatory effect on the gut mucosa. A number of research has showed a link between Fusobacterium genus and the development of colorectal cancer. Most recently an association between Fusobacterium nucleatum and colorectal cancer was established by a molecular cytogenetic technique[127,128].
Figure 3 The inhibitory properties of butyrate on tumorigenesis through various mechanisms by the colonic microbiota.
Many of the metabolites of protein fermentation can be taken up by other microorganisms and synthesized into active carcinogens. For example, amines and nitrates can be used by facultatively anaerobic and anaerobic colonic bacteria and catalysed the formation of N-nitrosamines, which are among the strongest procarcinogens. Reduced levels of butyrate in the body are not only an indication of the possibility of cancer, but also indicate the severity of the cancer and its course in the body.
The gut microbiota in patients with colorectal cancer is characterized by increased diversity in Clostridiaceae family (such as those from Clostridium genus), and an increase in Bacteroides and Bifidobacterium spp. In contrast, the gut microbiota of individuals with a reduced risk of developing cancer has a high population of lactate-producing bacteria, such as Eubacterium aerofaciens and Lactobacillaceae phyla[129]. Studies on animals led to propose a model of carcinogenesis based on the consideration that certain mutations in the intestinal epithelial cells lead to a loosening of the intercellular junctions and a reduced production of mucus, compromising the integrity of the intestinal mucosa. So, there is a translocation of bacteria from the lumen to the epithelium where microbial products bind to tumor-associated macrophage receptors inducing the release of inflammatory factors (such as IL-1, IL-6, IL-23) which in turn leads to the production of IL-17 stimulating T-helper lymphocytes. IL-17 activates the transcription factor signal transducer and activator of transcription 3 in epithelial cells which will lead to increased survival and proliferation of epithelial cells resulting in further mutations facilitating carcinogenesis[130,131]. These changes in the epithelium burden the already compromised epithelial integrity, exacerbating bacterial allostasis and contributing to the vicious cycle: Bacterial allostasis - inflammation - cancer. The IL-23/IL-17 axis in tumour-induced inflammation is caused by barrier loss. Thus, otherwise “good” bacteria in the gut microbiota can be transformed into a carcinogen due to the altered host defence mechanism, indicating the uniqueness of the mucosal environment in preventing the development of cancer and may can lead to a cancer progression in other sites such as make the gut/breast microbiota’s axis[132-135].