Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 21, 2023; 29(27): 4289-4316
Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4289
Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4289
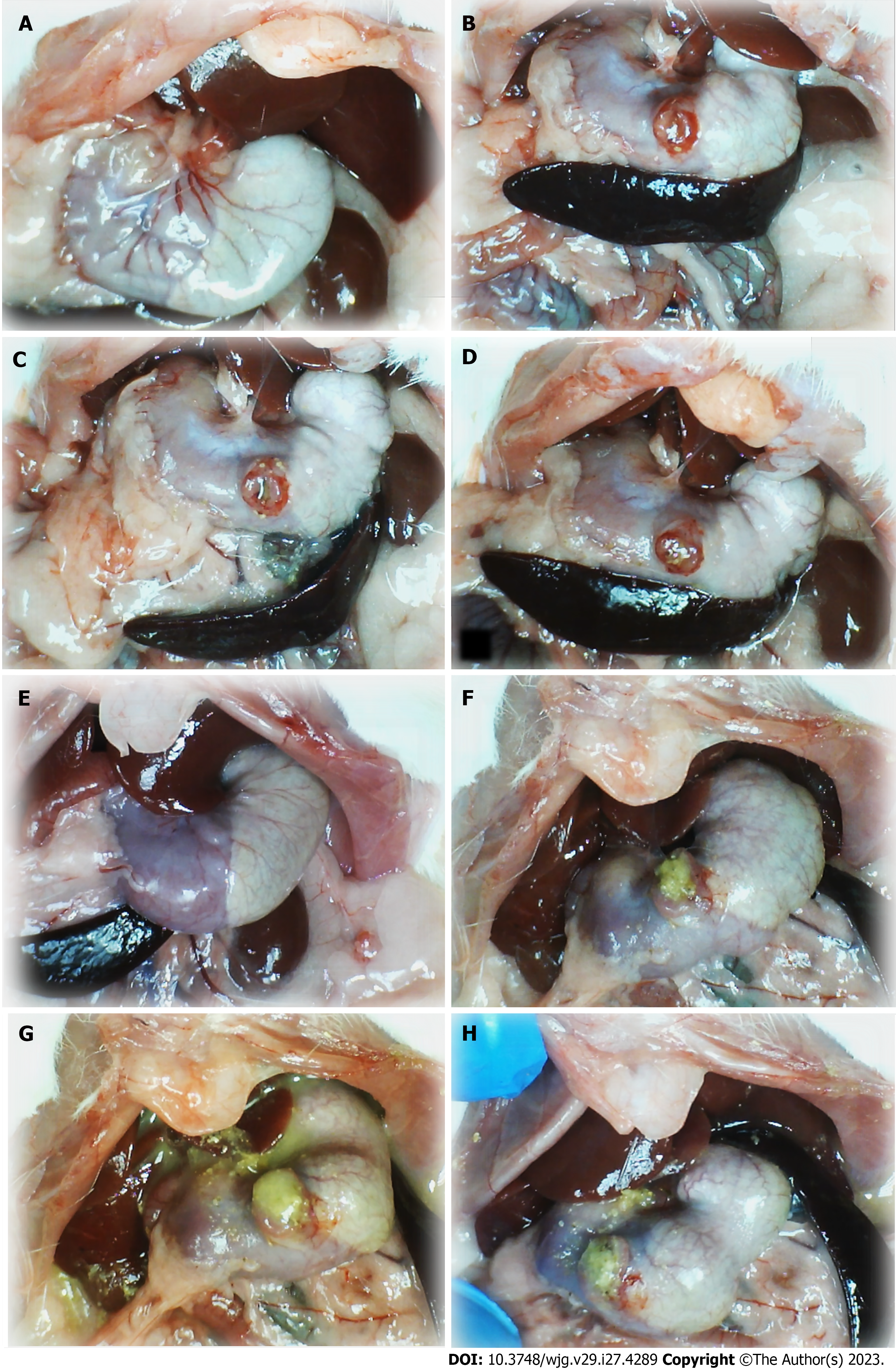
Figure 1 Stomach illustrative presentation.
A and E: Before procedure; B and F: After perforation before therapy; C and D: Course after given therapy into the perforated defect, saline; G and H: BPC 157. Control rats: (1) Before procedure (normal rats, A); (2) after perforation before therapy application (B); (3) after saline application (1 mL/rat into the perforated defect in the stomach) (C and D), immediately thereafter (C); and (4) later (at 5 min following medication, D). BPC 157 rats: (1) Stomach before perforation (normal rats, E); (2) after perforation, but before BPC 157 therapy application (F), and (3) specifically after BPC 157 application (10 µg or 10 ng/kg, 1 mL/rat into the perforated defect in the stomach, G and H), immediately thereafter (G), and later (at 5 min following medication, H).
- Citation: Kalogjera L, Krezic I, Smoday IM, Vranes H, Zizek H, Yago H, Oroz K, Vukovic V, Kavelj I, Novosel L, Zubcic S, Barisic I, Beketic Oreskovic L, Strbe S, Sever M, Sjekavica I, Skrtic A, Boban Blagaic A, Seiwerth S, Sikiric P. Stomach perforation-induced general occlusion/occlusion-like syndrome and stable gastric pentadecapeptide BPC 157 therapy effect. World J Gastroenterol 2023; 29(27): 4289-4316
- URL: https://www.wjgnet.com/1007-9327/full/v29/i27/4289.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i27.4289