Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3899
Peer-review started: April 9, 2023
First decision: May 12, 2023
Revised: May 20, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: June 28, 2023
Cirrhosis results from persistent liver injury that leads to liver fibrosis. Immunological factors play important regulatory roles in the development and pro
To provide a comprehensive overview of the knowledge structure and research hotspots of immunological factors in cirrhosis.
We retrieved publications related to immunological factors in cirrhosis between 2003 to 2022 from the Web of Science Core Collection database on December 7, 2022. The search strategy was TS = ((Liver Cirrhosis OR hepatic cirrhosis OR liver fibrosis) AND (Immunologic* Factor* OR Immune Factor* OR Immunomodulator* OR Biological Response Modifier* OR Biomodulator*)). Only original articles and reviews were included. A total of 2873 publications were analyzed using indicators of publication and citation metrics, countries, institutes, authors, journals, references, and keywords by CiteSpace and VOSviewer.
A total of 5104 authors from 1173 institutions across 51 countries published 2873 papers on cirrhosis and immunological factors in 281 journals. In the past 20 years, the increasing number of related annual publications and citations indicates that research on immunological factors in cirrhosis has become the focus of attention and has entered a period of accelerated development. The United States (781/27.18%), China (538/18.73%), and Germany (300/10.44%) were the leading countries in this field. Most of the top 10 authors were from the United States (4) and Germany (3), with Gershwin ME contributing the most related articles (42). World Journal of Gastroenterology was the most productive journal, whereas Hepatology was the most co-cited journal. Current research hotspots regarding immunological factors in cirrhosis include fibrosis, cirrhosis, inflammation, liver fibrosis, expression, hepatocellular carcinoma, activation, primary biliary cirrhosis, disease, and hepatic stellate cells. Burst keywords (e.g., epidemiology, gut microbiota, and pathways) represent research frontiers that have attracted the interest of researchers in recent years.
This bibliometric study comprehensively summarizes the research developments and directions of immunological factors in cirrhosis, providing new ideas for promoting scientific research and clinical applications.
Core Tip: Over the last 20 years, 5104 authors from 1173 institutions in 51 countries have published 2873 papers on cirrhosis and immunological factors in 281 journals. Immune dysfunction, gut microbiota, epidemiology, mouse model, pathway, obesity, extracellular vesicles, bile acids, and the extracellular matrix are currently at the frontiers of research on immunological factors in cirrhosis. It is notable that in addition to focusing on basic research, we should also pay attention to the translation and application of the research results.
- Citation: Zhang D, Liu BW, Liang XQ, Liu FQ. Immunological factors in cirrhosis diseases from a bibliometric point of view. World J Gastroenterol 2023; 29(24): 3899-3921
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3899
Hepatic cirrhosis is defined as extensive fibrosis, pseudolobules, and distortion of the hepatic vascular architecture due to various chronic liver diseases[1]. Patients with compensated cirrhosis are asymptomatic, whereas severely impaired liver function and portal hypertension are common in the decompensated stage. Patients with decompensated cirrhosis often have complications such as ascites, bleeding esophageal varices, hepatic encephalopathy, and hepatorenal syndrome, which may eventually progress to multi-organ failure and death. Cirrhosis has a diverse etiology and can be caused by chronic liver injury, progressing to inflammation and liver fibrosis with fibrous septa and regenerative nodules[2]. Subsequently, the liver structures may collapse, resulting in distortion of the liver parenchyma and vascular structures. Progressive fibrosis and cirrhosis subsequently lead to a decrease in the metabolic and synthetic functions of the liver, causing elevated bilirubin levels, decreased production of coagulation factors and thrombopoietins, splenic platelet retention, elevated portal vein pressure, and the development of ascites and esophageal varices[3]. Viral hepatitis [hepatitis B virus (HBV) infection and HCV infection], alcoholic and non-alcoholic fatty liver diseases are the most common identifiable causes[4].
Liver inflammation and immune responses are the key factors in the pathogenesis of cirrhosis. The immune system triggers inflammation during hepatic fibrosis and is involved in wound healing and tissue repair. Various cells in the liver, such as sinusoidal endothelial cells, intravascular liver-resident macrophages (Kupffer cells), and dendritic cells (DCs), are capable of antigen presentation and produce cytokines and chemokines[5]. Following liver injury, infiltrating immune cells promote the liver fibrosis cascade by secreting pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and chemokine 4 (CCL4). These cytoplasmic factors mediate interactions between immune cells and hepatic stellate cells (HSC), leading to HSC activation and fibroblast transdifferentiation. Immune-related genes are vitally important in immune infiltration and response[6]. These results indicate that immunological factors play a complex and important role in cirrhosis, and analysis of the results obtained in this area can help us estimate the trends of cirrhosis-related immunological factors and guide experimental strategies and funding decisions.
Bibliometrics, which applies mathematical and statistical methods to the study of books and other media, is one of the most commonly used methods for the systematic evaluation of a field of study. It can be used not only for qualitative and quantitative analysis in research on countries, regions, institutions, authors, co-cited authors, journals, references, and keywords, but also to describe and predict research hotspots and trends in specific fields[7]. Over the years, many scholars have applied bibliometric analyses to various fields of medicine, including cardiovascular diseases[8], respiratory diseases[9], obstetrical and gynecological diseases[10], psychiatric diseases[11], dermatological diseases[12], and biologic signaling[13]. To the best of our knowledge, bibliometric analyses of immunological factors in non-small cell lung cancer[14], cardiovascular diseases[15], and cancer[16] have been conducted by related scholars. Recent studies have shown that immunomodulators are a promising treatment option for cirrhosis. Therefore, we performed a bibliometric study of immunological factors in cirrhosis based on published data using CiteSpace and VOSviewer, two commonly used scientometric software packages, to create knowledge maps and explore the evolution and development trends of hotspots in this field. Microsoft Office Excel 2019 was used to perform a quantitative analysis of the publications and citations.
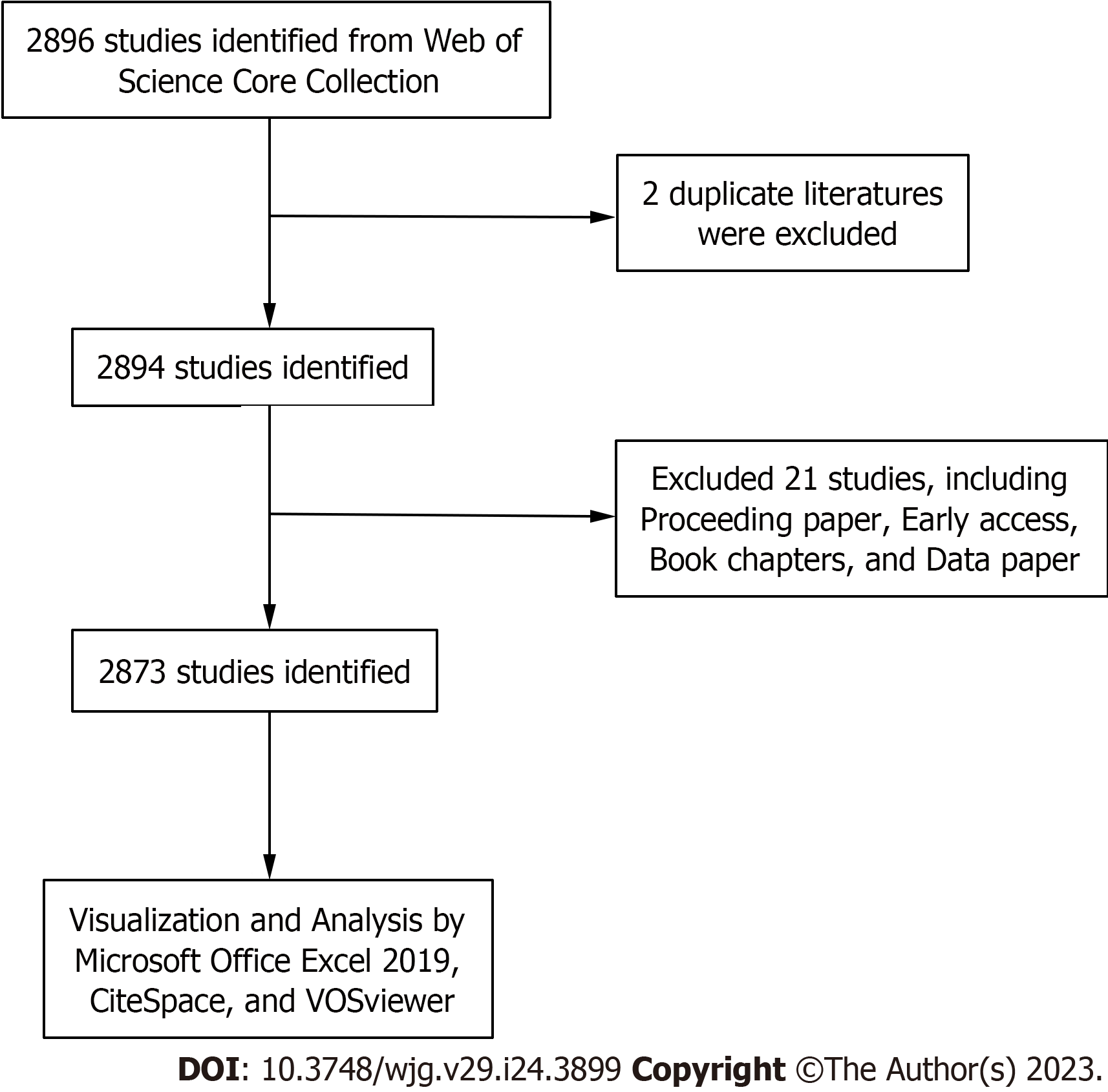
We used the Web of Science Core Collection (WoSCC) developed by Thomson Scientific to extract relevant literature on cirrhosis and immunological factors published between January 2003 and November 2022. To avoid citation fluctuations due to rapid updates, literature searches were conducted within 1 d (December 7, 2022). The search strategy was TS = ((Liver Cirrhosis OR hepatic cirrhosis OR liver fibrosis) AND (Immunologic* Factor* OR Immune Factor* OR Immunomodulator* OR Biological Response Modifier* OR Biomodulator*)). A total of 2896 publications was obtained. Among various publication types, only original articles and reviews were included. Finally, only 2873 publications that met the inclusion criteria were included in the bibliometric analyses. The detailed screening process is illustrated in Figure 1.
CiteSpace software was developed by Professor Chaomei Chen for bibliometric and visual analyses. Specifically, it explores collaborations, structures, hotspots, and frontiers in a research field and presents trends in a discipline or field of knowledge over time in a visual and intuitive form. Nodes with high centrality, highlighted by purple rings, are often considered hotspots or turning points in a research field. The thickness of the purple ring indicates the strength of its centrality; the thicker it is, the stronger its centrality[17]. Cluster analysis of keywords can be used to identify important regions of research on immunological factors in cirrhosis by classifying keywords. In addition, the variant word indicates a sudden growth of hotspots in the field and also shows the future trend of the topic[18]. A high mutation value indicated a large variation in size. Keywords and reference mutations can be used to identify new research trends in immunological factors associated with liver cirrhosis. Therefore, we used CiteSpace (version 6.1. R3) for the collaborative network analysis (country/region, institution, author, and journal), co-citation analysis (author, journal, and reference), dual mapping, and citation burst detection of references and keywords.
We constructed bibliometric maps using the VOSviewer software developed by Leiden University to obtain more comprehensive information about the results based on co-citation and co-occurrence. VOSviewer excels in map creation and presents bibliometric maps in different ways, including network, overlay, and density maps, each focusing on various aspects. The color of the nodes represents different times or clusters, the size of the nodes indicates the number of publications, and the thickness of the lines represents the strength of the relationship[19]. In our study, VOSviewer (version 1.6.18) performed the following analyses: Country and institution, authors, co-cited authors, and keywords.
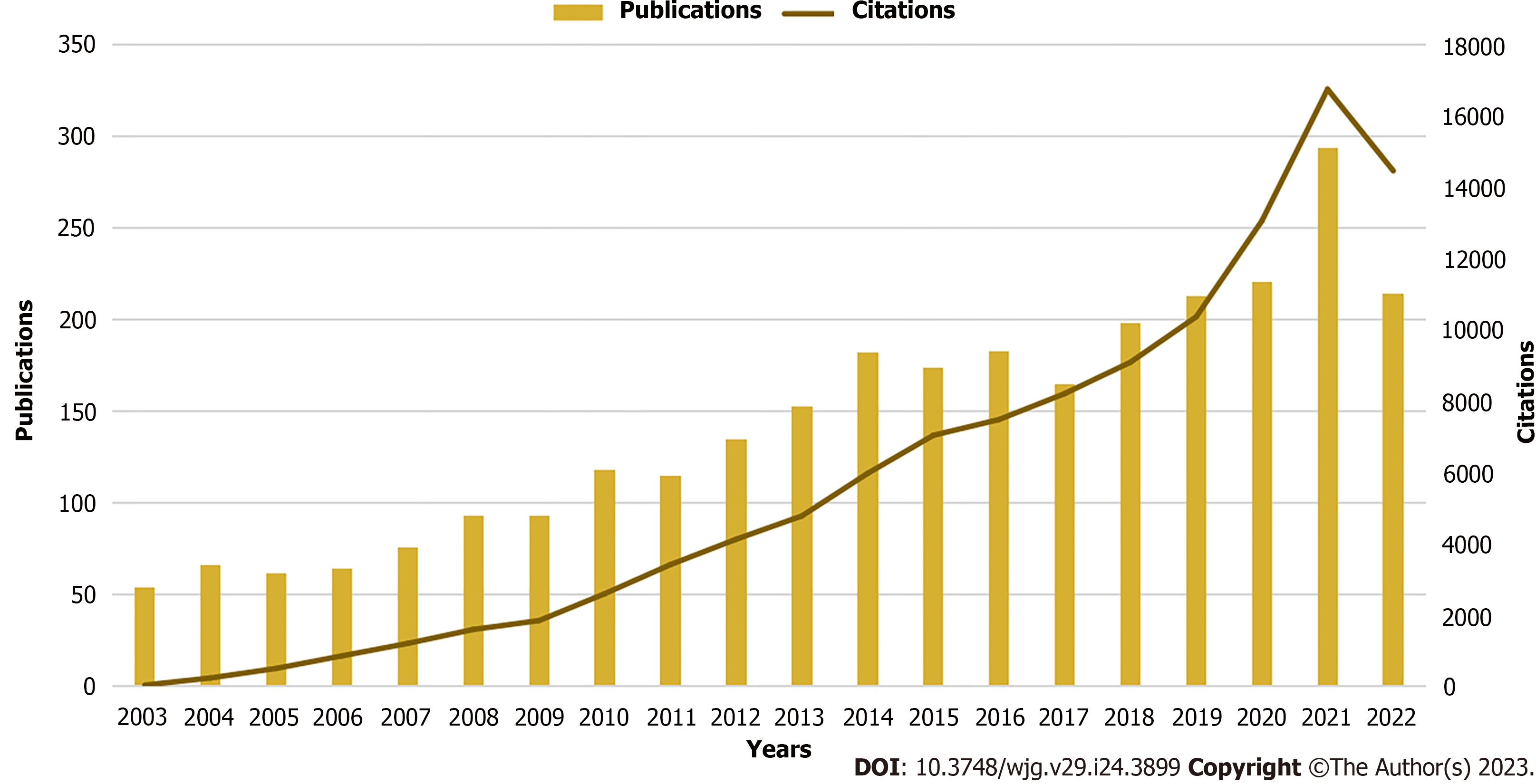
From 2003 to 2022, publications on immunological factors in cirrhosis included 1906 original articles and 967 reviews, showing a constantly increasing trend (Figure 2). From 2003 to 2014, the number of annual publications in this field steadily increased. Annual publications started with over 100 and 182 articles published in 2010 (n = 118) and 2014, respectively. Annual publications fluctuated slightly between 2014 and 2017 and gradually increased from 2018 to 2021. Subsequently, it increased significantly and peaked in 2021 (n = 294). The number of citations increased rapidly annually and exhibited a continuously rising trend. The upward trend in annual publications and citations indicates that research on immunological factors in cirrhosis has become a focus of attention and has entered a period of accelerated development.
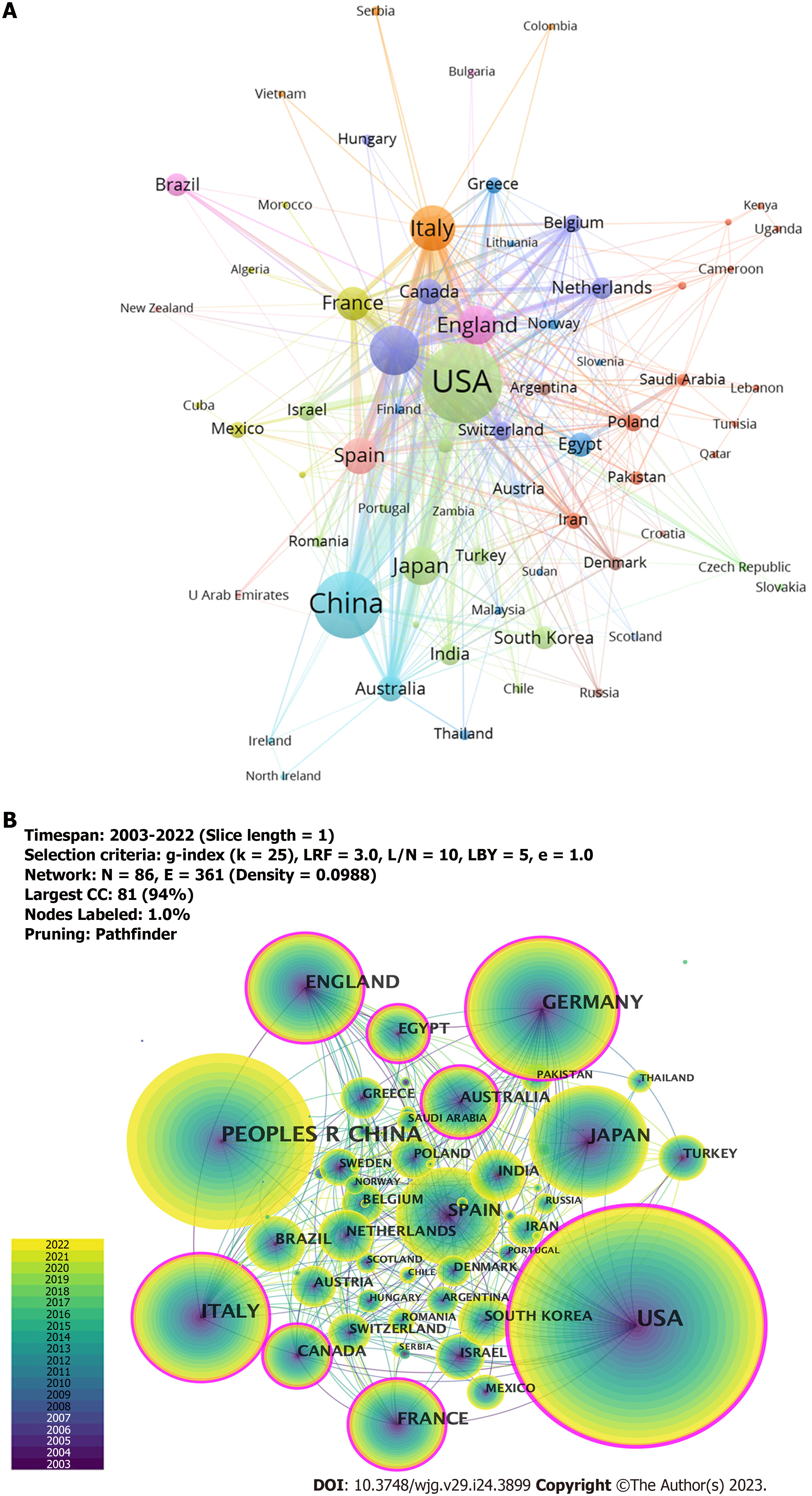
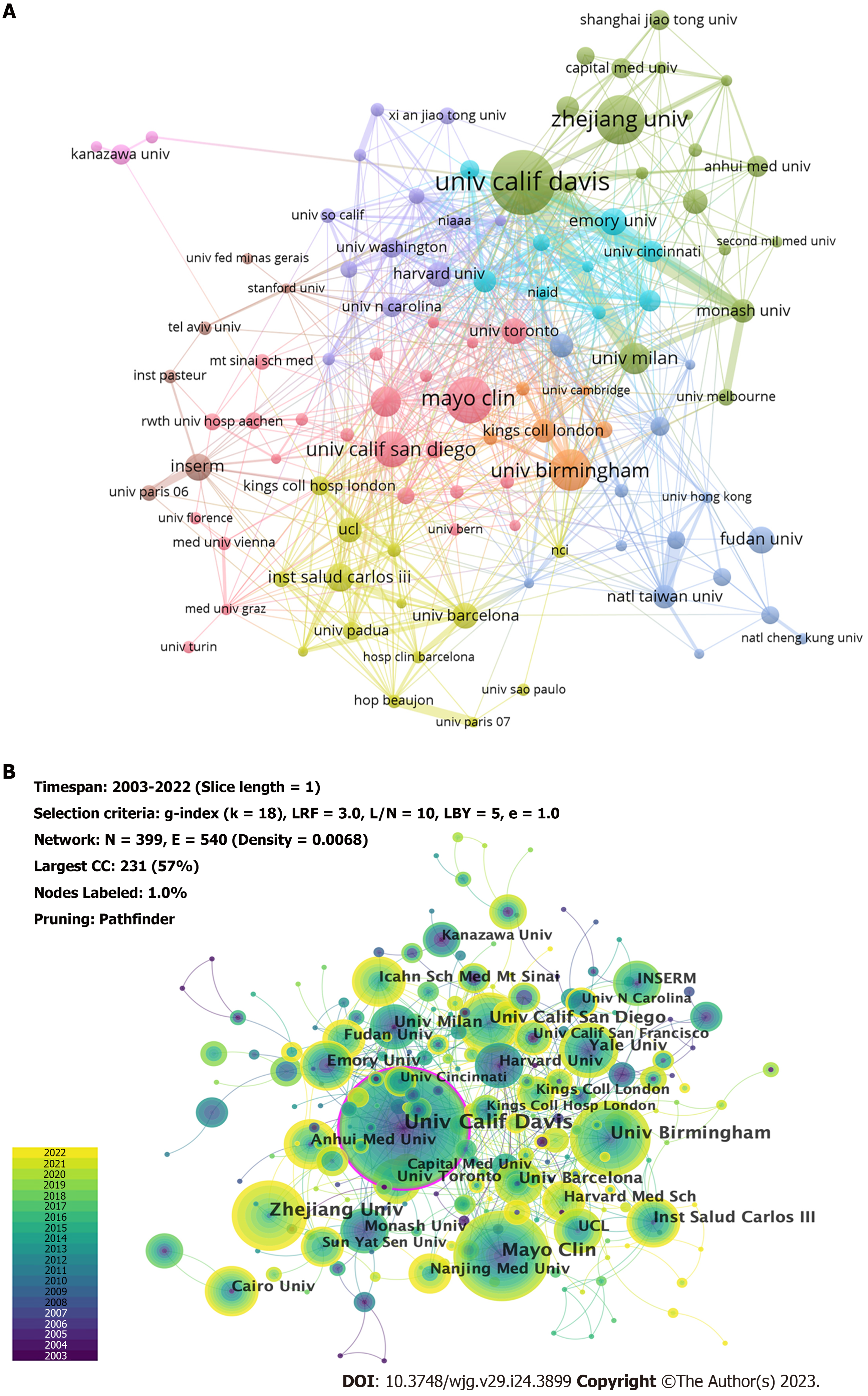
Publications were from 51 countries and 1173 institutions. As shown in Tables 1 and 2, we ranked the 10 high-productivity countries/regions and institutions. The United States (781/27.18%), China (538/18.73%), and Germany (300/10.44%) published the most articles, accounting for more than half of the total number of studies reported, indicating a high interest in studying immunological factors in cirrhosis. This was followed by Italy (259/9.01%) and Japan (192/6.68%). Subsequently, we constructed collaborative networks based on the publications and relationships in each country (Figure 3). It is noteworthy that there are many active collaborations among different countries (Figure 3A). For example, the United States has actively cooperated with China, Italy, Germany, England, Japan, France, and Australia; China has collaborated closely with the United States, Japan, Germany, Italy, England, Canada, and Australia; and Germany has collaborated with the United States, Switzerland, France, the Netherlands, Australia, China, and Spain. The top 10 institutions are distributed across five countries, with half in the United States. The University of California, Davis (65, 2.26%) led the publications, followed by the Mayo Medical Center (47, 1.64%) and Zhejiang University (44, 1.53%). We also observed a network diagram of institutional collaboration in research on immunological factors in cirrhosis (Figure 4A). The figure shows a very close cooperation among the University of California, Davis, University of Milan, Monash University, Emory University, and Mayo Clinic, and a more active collaboration between Zhejiang University, Emory University, and the University of Science and Technology of China. In addition, several countries and affiliations, including the United States (0.36), England (0.20), Italy (0.19), Germany (0.17), France (0.17), Canada (0.12), Australia (0.12), Egypt (0.11), and the University of California, Davis (0.12) showed high centrality, circled in purple in Figure 3B and Figure 4B, respectively. An article with high centrality could be a transformative discovery. These findings suggest that these countries and institutions play key roles in the study of immunological factors associated with cirrhosis. Notably, despite their high volume of publications, China and Japan might not provide scientists with the same level of novel insights compared to countries with high centrality.
| Rank | Country/region | Year | Count (%) | Centrality |
| 1 | United States | 2003 | 781 (27.18%) | 0.36 |
| 2 | China | 2003 | 538 (18.73%) | 0.06 |
| 3 | Germany | 2003 | 300 (10.44%) | 0.17 |
| 4 | Italy | 2003 | 259 (9.01%) | 0.19 |
| 5 | Japan | 2003 | 192 (6.68%) | 0.03 |
| 6 | England | 2003 | 186 (6.47%) | 0.20 |
| 7 | Spain | 2003 | 157 (5.46%) | 0.09 |
| 8 | France | 2003 | 132 (4.59%) | 0.17 |
| 9 | Australia | 2004 | 85 (2.96%) | 0.12 |
| 10 | Canada | 2003 | 84 (2.92%) | 0.12 |
| Rank | Institution | Year | Count (%) | Centrality |
| 1 | Univ Calif Davis (United States) | 2004 | 65 (2.26%) | 0.12 |
| 2 | Mayo Clin (United States) | 2007 | 47 (1.64%) | 0.02 |
| 3 | Zhejiang Univ (China) | 2011 | 44 (1.53%) | 0.01 |
| 4 | Univ Birmingham (England) | 2009 | 38 (1.32%) | 0.04 |
| 5 | Univ Calif San Diego (United States) | 2009 | 28 (0.97%) | 0.07 |
| 6 | Yale Univ (United States) | 2007 | 26 (0.90%) | 0.06 |
| 7 | Inst Salud Carlos III (Spain) | 2008 | 25 (0.87%) | 0.06 |
| 8 | Univ Milan (Italy) | 2004 | 24 (0.84%) | 0.06 |
| 9 | Univ Barcelona (Spain) | 2003 | 23 (0.80%) | 0.03 |
| 10 | Emory Univ (United States) | 2006 | 23 (0.80%) | 0.05 |
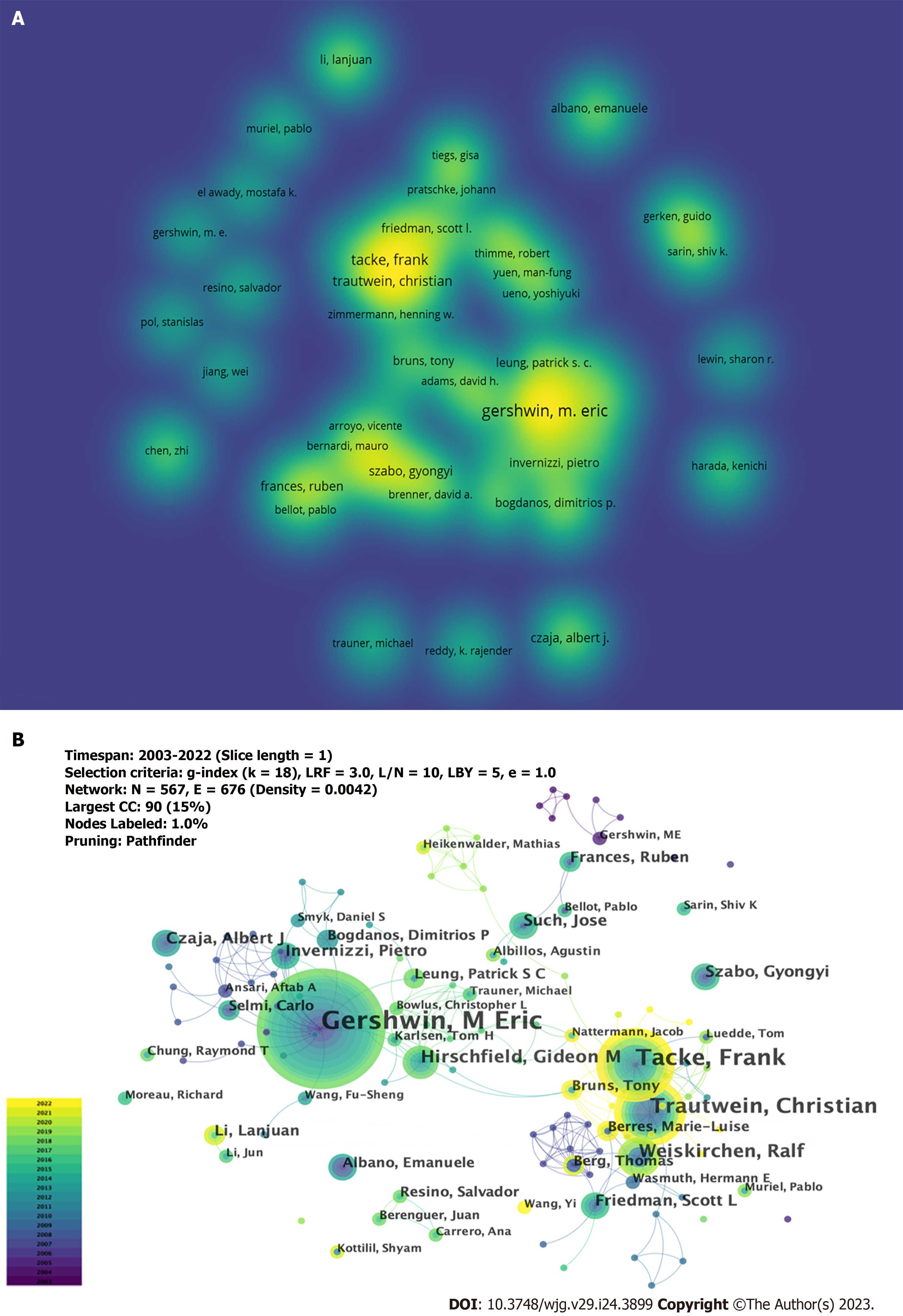
A total of 5104 authors participated in the study on the immunological factors associated with cirrhosis. The top 10 high-producing authors are listed in Table 3. As shown in Figure 5A, a larger font size and higher yellow opacity indicate more publications. Gershwin ME from the University of California Davis was the most productive author, with 42 publications, followed by Tacke et al[20] (n = 27) and Trautwein et al[21] (n = 20) from the University Hospital Aachen. Most of the top 10 authors were from the United States (4), and Germany (3). As shown in Figure 5B, there are collaborations between different authors; however, it is noteworthy that the centrality is low (≤ 0.02), which means that various authors have little influence on each other’s research. All the top 10 co-cited authors had more than 100 co-citations (Table 3). As shown in Figure 6A, a larger font size and a higher yellow opacity imply a higher citation frequency. Friedman, from the Icahn School of Medicine, had the highest number of co-citations (n = 337). The next most cited were Bataller (n = 235) from Pittsburgh Liver Research and Seki (n = 204) from the Cedars-Sinai Medical Center. More than half (n = 6) of the top 10 co-cited authors were from the United States. As shown in Figure 6B, there was active cooperation between the different authors.
| Rank | Author | Count (%) | Centrality | Co-cited author | Co-citation | Centrality |
| 1 | Gershwin M Eric (United States) | 42 (1.46%) | 0.02 | Friedman Scott L (United States) | 337 | 0.05 |
| 2 | Tacke Frank (Germany) | 27 (0.94%) | 0.01 | Bataller Ramon (United States) | 235 | 0.06 |
| 3 | Trautwein Christian (Germany) | 20 (0.70%) | 0.01 | Seki Ekihiro (United States) | 204 | 0.05 |
| 4 | Weiskirchen Ralf (Germany) | 13 (0.45%) | 0 | Tilg Herbert (Austria) | 141 | 0.07 |
| 5 | Hirschfield Gideon M (Canada) | 11 (0.38%) | 0.01 | Wynn Tomas A (United States) | 140 | 0.02 |
| 6 | Friedman Scott L (United States) | 9 (0.31%) | 0 | Albillos Agustín (Spain) | 128 | 0.01 |
| 7 | Such Jose (Spain) | 9 (0.31%) | 0 | Szabo Gyongyi (United States) | 126 | 0.04 |
| 8 | Szabo Gyongyi (United States) | 8 (0.28%) | 0 | Gao Bin (United States) | 125 | 0.08 |
| 9 | Invernizzi Pietro (Italy) | 8 (0.28%) | 0 | Hirschfield Gideon M (Canada) | 120 | 0.09 |
| 10 | Czaja Albert J (United States) | 8 (0.28%) | 0 | Li J (China) | 116 | 0.02 |
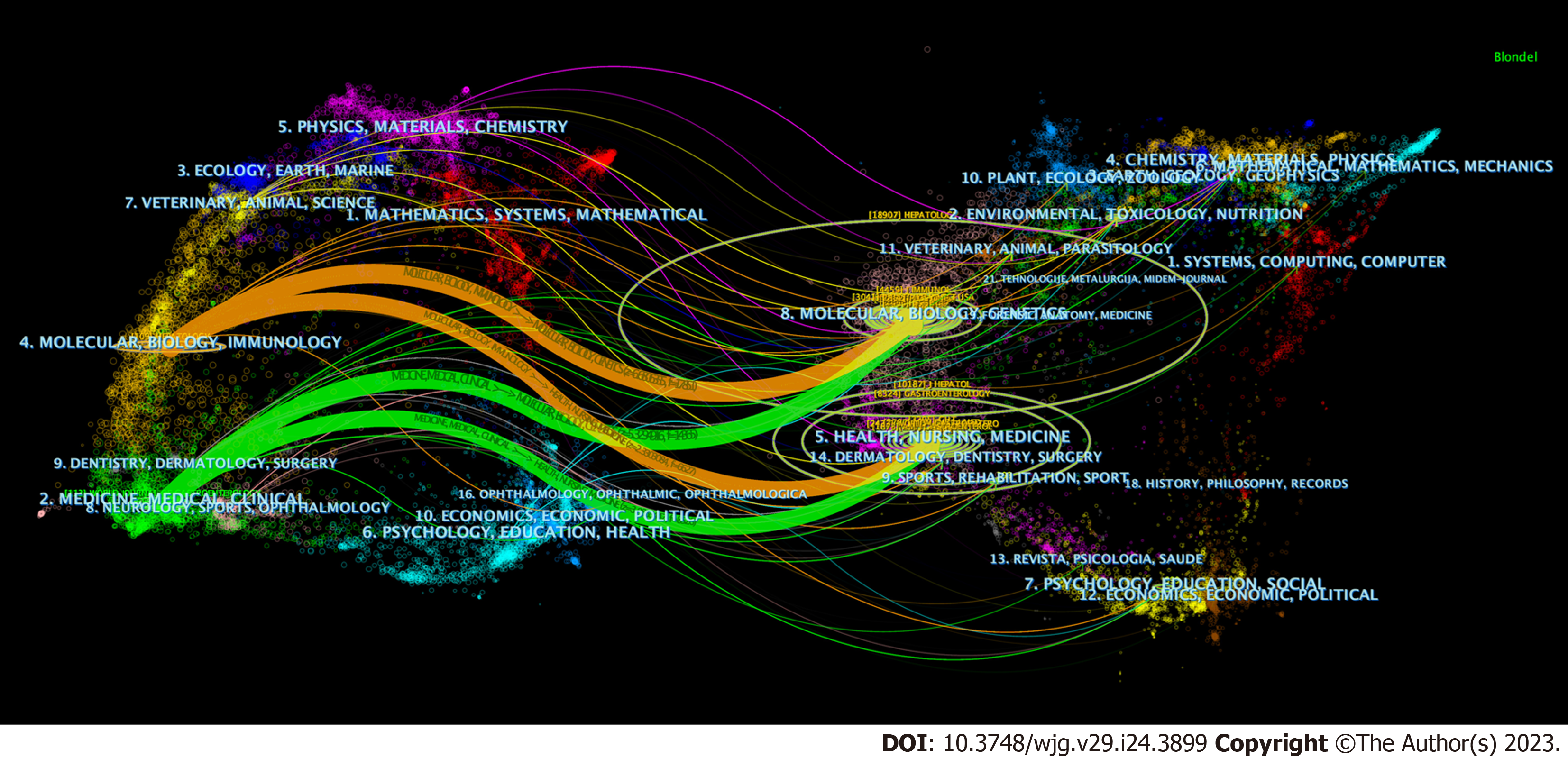
The most influential journals on research on immunological factors in cirrhosis are listed in Table 4. A total of 2873 articles were published in 281 journals. The World Journal of Gastroenterology was the most frequently published journal (n = 113), followed by Hepatology (n = 109) and Frontiers in Immunology (n = 73). Among the top 10 journals, six belonged to Journal Citation Reports (JCR) Q1, and eight had impact factors (IF) of > 5. Among the co-cited journals, 12 had > 1000 citations. Hepatology was ranked first with 2409 citations, followed by the Journal of Hepatology (2124 citations) and Gastroenterology (1988 citations). Eight of the top 10 co-cited journals belonged to JCR Q1, and eight journals had IF > 10, including two journals with IF above 100. We analyzed the published and co-cited journals using a journal dual-map overlay, as shown in Figure 7. The cited and co-cited journals are located on the left and right sides of the graph, respectively. Different colored paths indicate the cited relationships. We identified two orange and green citation paths. Studies published in the Molecular/Biology/Clinics and Health/Nursing/Medicine journals were mainly cited in the Molecular/Biology/Immunology and Medicine/Medical/Clinical journals.
| Rank | Journal | Count (%) | IF (2021) | JCR | Co-cited Journal | Citation | IF (2021) | JCR |
| 1 | World Journal of Gastroenterology | 113 (3.928%) | 5.374 | Q2 | Hepatology | 2409 | 17.298 | Q1 |
| 2 | Hepatology | 109 (3.790%) | 17.298 | Q1 | Journal of Hepatology | 2124 | 30.083 | Q1 |
| 3 | Frontiers in immunology | 73 (2.540%) | 8.786 | Q1 | Gastroenterology | 1988 | 33.883 | Q1 |
| 4 | Journal of Hepatology | 70 (2.430%) | 30.083 | Q1 | Gut | 1369 | 31.793 | Q1 |
| 5 | Liver International | 59 (2.050%) | 8.754 | Q1 | Journal Of Clinical Investigation | 1301 | 19.456 | Q1 |
| 6 | PLoS One | 57 (1.980%) | 3.752 | Q2 | Journal Of Immunology | 1280 | 5.426 | Q2 |
| 7 | International Journal of Molecular Sciences | 46 (1.600%) | 6.208 | Q1 | The New England Journal of Medicine | 1240 | 176.079 | Q1 |
| 8 | Gastroenterology | 35 (1.220%) | 33.883 | Q1 | Proceedings of the National Academy of Sciences of the United States of America | 1221 | 12.779 | Q1 |
| 9 | Seminars in Liver Disease | 32 (1.110%) | 6.512 | Q2 | PLoS One | 1183 | 3.752 | Q2 |
| 10 | Hepatology Research | 30 (1.040%) | 4.942 | Q2 | Lancet | 1153 | 202.731 | Q1 |
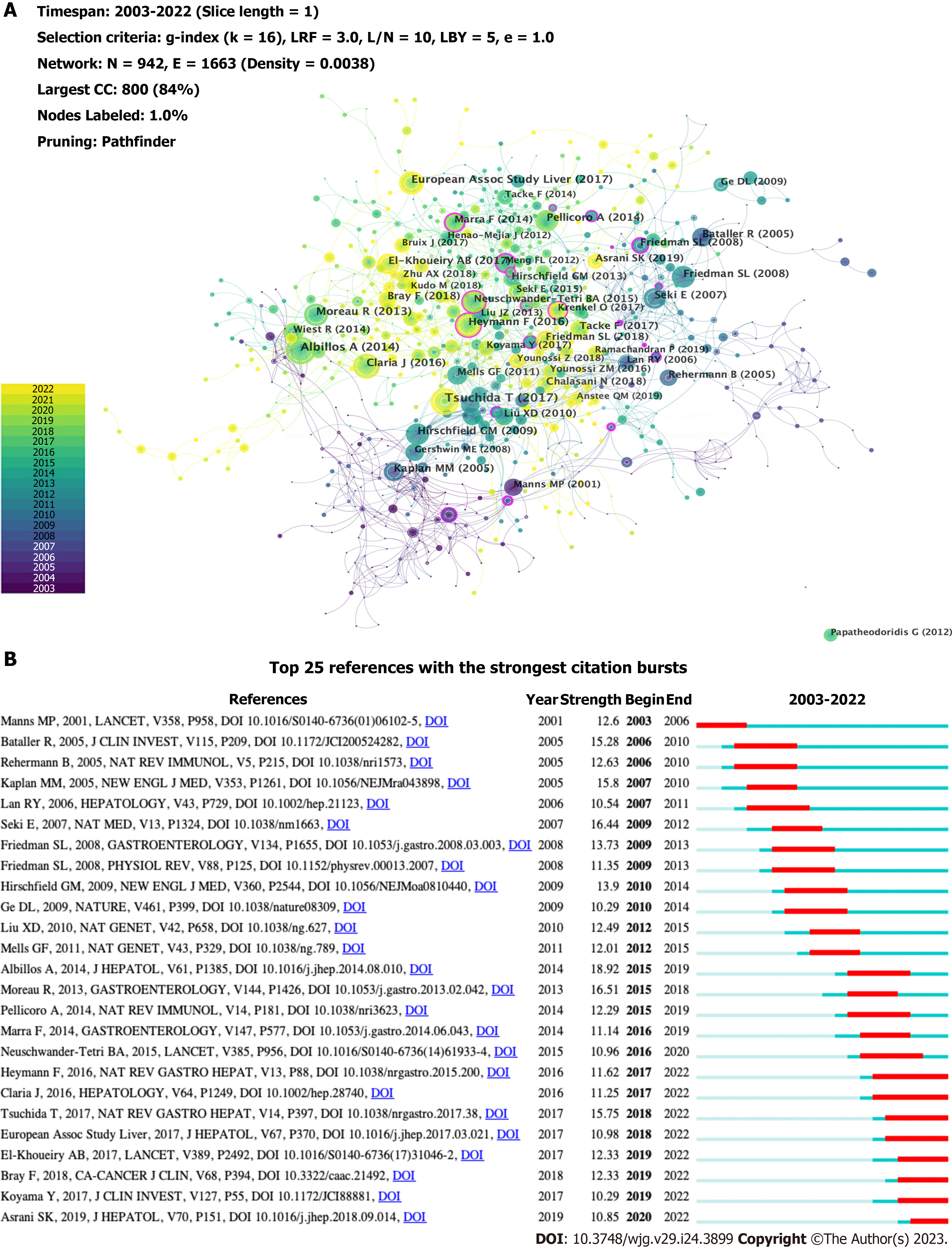
The top 10 co-cited references are listed in Table 5, all of which were co-cited at least 31 times. A reference co-citation network was mapped (Figure 8A). The link between two nodes implies that they are cited in a publication. The node size indicates the co-citations of the references. A node with a purple ring represents a higher centrality. The article with the most co-citations was by Tsuchida et al[22], which revealed that HSC activation is regulated by multiple mechanisms, including autophagy, endoplasmic reticulum stress, oxidative stress, retinol and cholesterol metabolism, epigenetic and receptor-mediated signaling, and extracellular signals from resident and inflammatory cells, which play an essential role in the development of liver fibrosis. The second study, published by Albillos et al[23] in 2014, revealed that immunodeficiency in cirrhosis is induced by local immune surveillance, functional impairment of the liver, reduction of pattern recognition receptors, and damage at the systemic level of immune response cell function. The third article[24] published in the Journal of Hepatology by the European Association for the Study of the Liver, provided validated recommendations for the optimal management of HBV infection. Among the top 10 articles, research by Heymann et al[5] possessed a high centrality (0.14), indicating that the article is influential. It illustrated that the liver can rapidly activate immunity, and the final outcome of the intrahepatic immune response depends on the functional versatility of macrophages and DCs and the balance between the pro- and anti-inflammatory T cell populations. CiteSpace provides citation bursts that imply significant changes in reference citations over time. References with citation bursts have frequently been cited by researchers in recent years to represent emerging topics in specific research field[25]. Figure 8B shows the 25 most cited references with the strongest bursts. The most-cited study was by Albillos et al[23], with a strength of 18.92. Tsuchida et al[22] also reported a large burst. These are representative articles on the immunological factors involved in cirrhosis.
| Rank | Reference | Year | Journal | Citation | Centrality |
| 1 | Mechanisms of hepatic stellate cell activation | 2017 | Nature Reviews Gastroenterology & Hepatology | 50 | 0.03 |
| 2 | Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance | 2014 | Journal of Hepatology | 46 | 0.02 |
| 3 | EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection | 2017 | Journal of Hepatology | 35 | 0.03 |
| 4 | Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis | 2013 | Gastroenterology | 35 | 0.03 |
| 5 | Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries | 2018 | CA: A Cancer Journal for Clinicians | 33 | 0.02 |
| 6 | Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial | 2017 | Lancet | 33 | 0.04 |
| 7 | Immunology in the liver-from homeostasis to disease | 2016 | Nature Reviews Gastroenterology & Hepatology | 32 | 0.14 |
| 8 | Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure | 2016 | Hepatology | 31 | 0.07 |
| 9 | Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants | 2009 | The New England Journal of Medicine | 31 | 0.02 |
| 10 | TLR4 enhances TGF-beta signaling and hepatic fibrosis | 2007 | Nature Medicine | 31 | 0.06 |
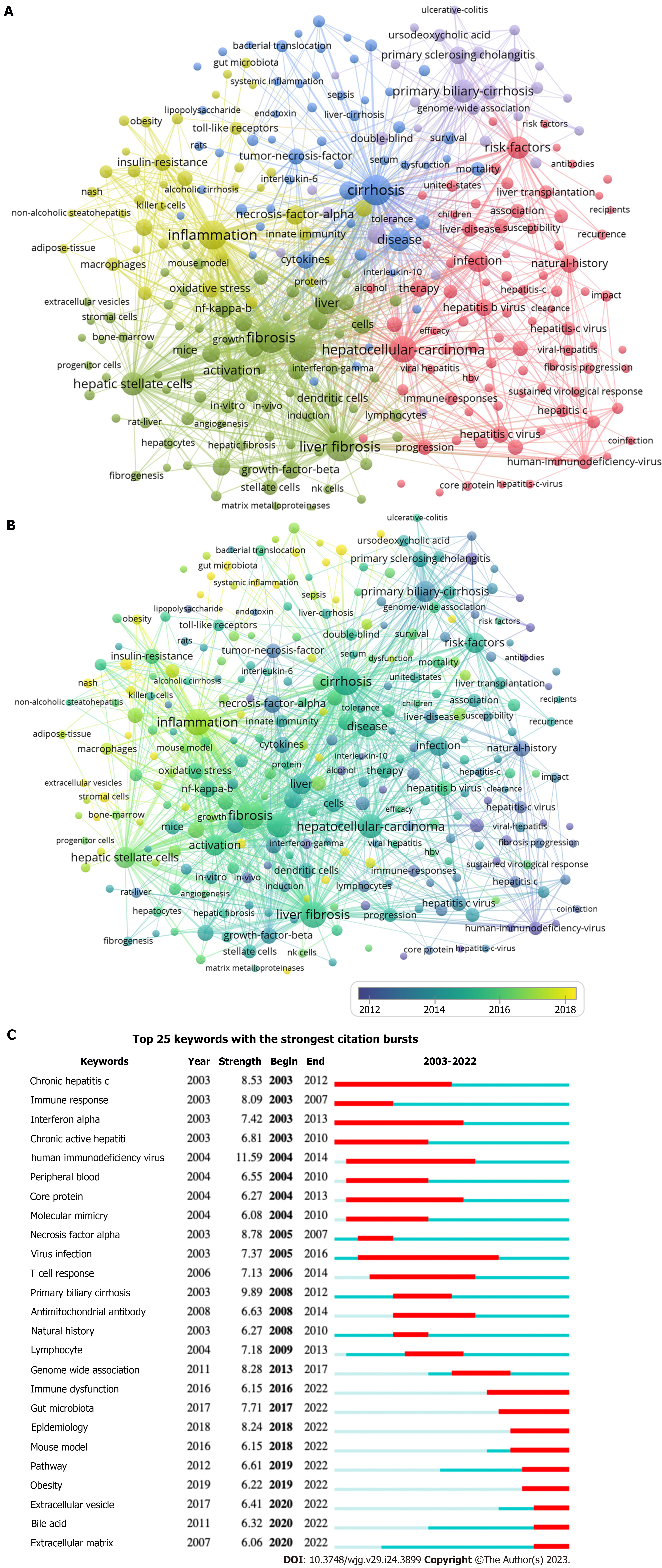
As shown in Table 6, the most frequently cited keywords for immunological factors in cirrhosis in the last 20 years were fibrosis (428), cirrhosis (423), inflammation (384), liver fibrosis (381), expression (340), hepatocellular carcinoma (HCC) (309), activation (234), primary biliary cirrhosis (PBC) (232), disease (231), and HSC (228). These keywords reflect the research hotspots in this field. Among the 10689 keywords, the minimum number of repetitions was set to 20 and 288 eligible keywords were selected for VOSviewer visualization and analysis (Figure 9). The keyword clusters describe the internal knowledge structure of the research field[7]. The immunological factors associated with cirrhosis were divided into five clusters (Figure 9A). Cluster 1 (red) focused on the progression and treatment strategies for cirrhosis. Cluster 2 (green) includes the mechanisms and primary immune cells involved in cirrhosis. Cluster 3 (blue) mainly focused on the diagnosis and prognosis of cirrhosis. Cluster 4 (yellow) centered on the pathological mechanisms of immune inflammation. Cluster 5 (purple) mainly consisted of various chronic liver diseases that cause liver injury. Figure 9B shows the co-occurrence of keywords by the average publication year (APY). Circles in different colors indicate the APY of the study, and those closer to yellow represent more recent years. Therefore, changes in research hotspots during a given period can be observed directly. The most recent keywords were extracellular vesicles (EVs) (cluster 2, APY: 2019.55), followed by tumor microenvironment (cluster 2, APY: 2019.43), PBC (cluster 5, APY: 2019.33), nafld (cluster 4, APY: 2019.19), and gut microbiota (cluster 4, APY: 2019.17). This indicates that EVs, the tumor microenvironment, PBC, nonalcoholic fatty liver disease, and gut microbiota have interested researchers. Additionally, we used CiteSpace for keyword–citation burst detection. There are 25 keywords had strong bursts, as shown in Figure 9C. Human immunodeficiency virus ranked first, with the strongest burst (strength = 11.59). Immune dysfunction, gut microbiota, epidemiology, mouse models, pathways, obesity, EVs, bile acids, and the extracellular matrix (ECM) are the current research frontiers of immunological factors in cirrhosis and have been in an explosive period.
| Rank | Keyword | Occurrences | Total link strength | Rank | Keyword | Occurrences | Total link strength |
| 1 | Fibrosis | 428 | 2558 | 11 | Liver | 225 | 1276 |
| 2 | Cirrhosis | 423 | 2483 | 12 | Risk factors | 221 | 1200 |
| 3 | Inflammation | 384 | 2438 | 13 | Infection | 184 | 1123 |
| 4 | Liver fibrosis | 381 | 2290 | 14 | Necrosis factor alpha | 160 | 1001 |
| 5 | Expression | 340 | 1884 | 15 | T cells | 155 | 923 |
| 6 | Hepatocellular carcinoma | 309 | 1895 | 16 | Cells | 143 | 770 |
| 7 | Activation | 234 | 1421 | 17 | Regulatory t cells | 140 | 863 |
| 8 | Primary biliary cirrhosis | 232 | 1268 | 18 | Liver cirrhosis | 133 | 770 |
| 9 | Disease | 231 | 1355 | 19 | Growth factor beta | 130 | 834 |
| 10 | Hepatic stellate cells | 228 | 1525 | 20 | Oxidative stress | 129 | 836 |
According to the WoSCC database, as of December 7, 2022, 5104 authors from 1173 institutions in 51 countries have published 2873 papers on cirrhosis and immunological factors in 281 journals. Our study analyzed the literature on immunological factors in cirrhosis using CiteSpace and VOSviewer to review research results and progress. Basic information, including annual publications, countries, institutions, authors, journals, references, and keywords, was quantitatively analyzed. There is a gradual increase in publications and citations on the immunological factors of cirrhosis. More citations of a paper indicate higher quality and greater impact. The number of citations in the field has increased annually, with an accelerating trend in the later half of the period reflecting the speed and progress of the research field. This indicates that research in this field has been in an explosive period, with increasing attention from scholars.
The United States, China, and Germany are the main countries that have studied the immunological factors associated with cirrhosis. Japan, Australia, France, and United States have relatively well-researched studies on immunological factors in cirrhosis. Of the top 10 institutions, five are in the United States, two in Spain, and one each in China, England, and Italy. Davis University of California has the most publications in this field. Close collaboration between countries and institutions helps to overcome scientific difficulties and promote research on immunological factors in cirrhosis.
Gershwin ME from the United States published the most articles (42, 1.46%), followed by Tacke et al[20] (27, 0.94%) and Trautwein et al[21] (20, 0.70%) from Germany. These three authors have made outstanding contributions to the field of immunological factors in cirrhosis. Gershwin explained the immune response mechanisms in the liver[26] and elucidated the regulatory mechanisms of liver fibrosis from an immunological perspective[27,28]. They then explored the relationship between the gut microbiota and autoimmune liver disease and concluded that the gut-liver axis involves multiple inflammatory cell types, cytokines, chemokines, and other molecules that lead to the disruption of normal liver structure[29]. Tacke et al[20] researched the role of macrophages in liver fibrosis, the polarization and function of which can be further determined by specific environmental signals, presenting with tissue recovery following liver injury or infection, or the progression of liver disease, conversely[30]. They found that infiltrating Ly-6C+ MoMFs are associated with chronic inflammation and fibrosis[20]. They considered hepatic macrophages to be effective therapeutic targets and searched for new treatments[31]. Two study elaborated on the importance of chemokines in liver diseases and highlighted their potential to intervene in inflammation and fibrosis[32,33]. They proposed that canicriviroc treatment significantly reduced hepatic Ly-6C+ MoMF recruitment, confirming its therapeutic potential in patients with nonalcoholic steatohepatitis (NASH)[34]. They also found that NOD-like receptor protein 3 activation was relevant to disease activity in patients with PBC[35].
Of the top 10 journals, six were in the Q1 JCR division and eight in the IF > 5. Most studies on immunological factors in cirrhosis were published in the World Journal of Gastroenterology (113, IF = 5.374, Q2), followed by Hepatology (109, IF = 17.298, Q1), and Frontiers in Immunology (73, IF = 8.786, Q1). These journals are the most popular in this research field. Eight of the top 10 co-cited journals were located in the Q1 JCR division, and eight had IF above 10, including two journals with IF above 100. The top three co-cited journals were Hepatology (2409, IF = 17.298, Q1), Journal of Hepatology (2124, IF = 30.083, Q1), and Gastroenterology (1988, IF = 33.883, Q1). These findings indicate that many high-quality and influential journals are interested in studying cirrhosis and its immunological factors. Figure 7 reveals that papers published in molecular, biology, clinical, health, nursing, and medicine journals are mainly cited in Molecular, Biology, Immunology, and Medicine, Medical, Clinical journals. This implies that current research on cirrhosis and immunological factors is mainly focused on basic research and translational medicine.
We can observe the research base in the field of immunological factors in liver cirrhosis by analyzing the co-cited references. The most co-cited was “Mechanisms of hepatic stellate cell activation” published in Nature Reviews Gastroenterology & Hepatology by Tsuchida et al[22] in 2017, followed by “Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance” by Albillos et al[23], and “EASL 2017 Clinical Practice Guidelines on the management of HBV infection” by European Association for the Study of the Liver. The top 10 co-cited references were all cited 30 times at least, mainly involving the immunoinflammatory mechanisms of cirrhosis and liver fibrosis. Liver fibrosis is a dynamic process caused by chronic liver injury. The inflammatory response in the liver is initiated by danger-associated molecular patterns, Toll-like receptors (TLRs) signaling, or inflammatory vesicle activation[5]. HSC activation is the central driver of liver fibrosis[22]. The activation of hepatic macrophages (Kupffer cells) and resident mast cells promotes tissue injury by producing pro-inflammatory and vascular-permeable mediators, which induce the accumulation of neutrophils, lymphocytes, eosinophils, and monocytes in the liver[36]. Systemic inflammation, the main driver of acute-on-chronic liver failure (ACLF), affects the function of somatic tissues and alters the clinical manifestations of cirrhosis[37]. The immune response pattern changes from a proinflammatory phenotype in patients with stable decompensated cirrhosis to an immunodeficient phenotype in patients with severely decompensated cirrhosis and extrahepatic organ failure[23].
In the study of immunological factors in cirrhosis, high-frequency keywords were mainly fibrosis (428), cirrhosis (423), inflammation (384), liver fibrosis (381), expression (340), HCC (309), activation (234), PBC (232), disease (231), and HSC (228), which are the hot topics of research in this field. Systemic inflammation is a crucial mechanism in the progression of cirrhosis to the decompensated stage and is a common pathophysiological mechanism underlying the clinical features of acutely decompensated cirrhosis, such as ascites, encephalopathy, gastrointestinal bleeding, bacterial infection, and extrahepatic organ failure[38]. IL-6, TNF-α, and transforming growth factor beta (TGF-β) are key pro-inflammatory and pro-fibrotic cytokines that drive liver fibrosis[39]. A recent study found that under pathological conditions, TGF-β overexpression leads to epithelial mesenchymal-mesenchymal transition, ECM deposition, and cancer-associated fibroblast formation, resulting in fibrotic disease and cancer[40]. Toxins such as alcohol, steatosis, or viral infection can trigger inflammation, and the inflammatory cytokine IL-17 drives HSC and Kupffer cells to produce pro-fibrotic IL-6, TNF-α, and TGF-β[41]. A previous study[42] demonstrated that albumin binds and activates many inflammatory promoters in decompensated cirrhosis and that high doses of albumin have significant immunomodulatory effects. However, the anti-inflammatory effects of albumin in cirrhosis require further investigation. This evidence suggests the potential value of anti-inflammatory agents in the treatment of cirrhosis. Approximately 90% of HCC cases occur in a cirrhotic setting, but less than 5% of patients progress to HCC each year[43]. Cancer-associated fibroblasts, which may originate from HSC, are critical for hepatocarcinogenesis. After being activated to become myofibroblasts producing the ECM, they can interact with cancer cells, contributing to the growth and invasion of HCC[44]. PBC is characterized by a loss of immune tolerance in biliary epithelial cells, and the interdependence of biliary tract injury, cholestasis, and progressive hepatic fibrosis has become a major disease worldwide[45]. PBC is considered an autoimmune disease with a specific autoantibody, antimitochondrial antibody, and the establishment of ursodeoxycholic acid as a first-line therapeutic agent has significantly reduced disease progression in cirrhosis[46]. In patients with PBC who did not respond adequately to ursodeoxycholic acid alone, besides ursodeoxycholic acid, we also treated them with bezafibrate; however, its effect on liver transplantation and death needs to be clarified by further studies[47]. Liver fibrosis is an advanced stage of chronic liver disease that develops from a variety of diseases, such as chronic hepatitis virus infection (HBV and HCV), NASH, PBC, primary sclerosing vasculitis, autoimmune hepatitis, Wilson’s disease, and hemochromatosis[48]. Researches on the pathogenesis and targeted agent of PBC have excited particular concern in recent years[49,50]. The crucial role and activation mechanisms of HSC in cirrhosis have been extensively elaborated in detail[22,51,52]. Paracrine signals from damaged epithelial cells, fibrotic microenvironment, dysregulation of immune and systemic metabolism, dysregulated intestinal microecology, and hepatitis virus products can directly or indirectly activate HSC[51]. The clearance of HSC, including apoptosis, senescence, and return to an inactivated state, has been fully elucidated[22]. In conclusion, these findings reveal the remarkable complexity and plasticity of HSC activation. The modulation of HSC provides vital value for developing therapeutic strategies for cirrhosis.
The keywords that have exploded in recent years are immune dysfunction, gut microbiota, epidemiology, mouse models, pathways, obesity, EVs, bile acid, and ECM, representing the current frontiers of research on immunological factors in cirrhosis. In hepatic fibrosis, the immune system is involved in wound healing and tissue repair by triggering inflammation. Immune cells regulate the progression and regression of liver fibrosis. Various immune cells participate in the regulation of liver fibrosis, including innate immune cells (monocytes, macrophages, and DCs), adaptive immune cells (T cells and B cells), and various cytokines. Following liver injury, infiltrating immune cells are recruited to the site of the injured hepatocytes and participate in the liver fibrosis cascade by secreting proinflammatory cytokines such as TNF-α, IL-6, and CCL4[39]. As the first line of defense against pathogens, Kupffer cells are activated by LPS, the complement system, and other pathogen-associated molecular patterns via the expression of TLRs, including TLR2, TLR3, and TLR4, to recognize microbial antigens and signals from damaged hepatocytes. Cytoplasmic division generated by Kupffer cells in response to TLR signaling subsequently recruits and activates neutrophils[6]. DCs present antigens, control subsequent T cell differentiation, and regulate T cell responses[53]. Systemic inflammation and immunodeficiency have been identified as two critical factors in cirrhosis-associated immune dysfunction, with the most severe immune changes being more common in ACLF[54]. The pathophysiology of ACLF involves persistent inflammation, immune dysregulation, systemic inflammatory response syndrome, and subsequent sepsis due to immune paralysis[55,56]. Two main types of systemic immune alterations are typical in cirrhosis: On the one hand, cirrhosis-associated immune dysfunction results in immunodeficiency; on the other hand, persistent under-stimulation of immune cells leads to systemic inflammation[53]. Immunodeficiency in cirrhosis results from the structural distortion of the liver parenchyma and functional impairment of circulating immune cells. It can increase the risk of bacterial infection at any stage of liver disease and may lead to acute decompensation and acute or chronic liver failure, which are associated with high short-term mortality[36,37]. Inflammatory factors play an essential role in immune regulation. Further regulation of relevant immune cells and cytokines to control inflammation and block critical targets of the immune-inflammatory response can delay and inhibit the onset and progression of cirrhosis. Gut microbiota is involved in maintaining the balance of the immune system and preventing autoimmunity[57]. In chronic liver disease, the adhesion of pathogenic bacteria to mucosal surfaces and barrier damage are central to the initiation of mucosal immunity[58]. Microbial products (especially LPSs), as pathogen-associated molecular patterns, can be transferred from the gut to the liver, bind to TLR, activate Kupffer cells, stimulate immune responses, and produce inflammatory cytokines (e.g., IL-1β and TNF) and reactive oxygen species[57,59]. The gut microbiota can lead to systemic inflammation either directly through translocation or indirectly through its metabolic pathways, resulting in the progression of de-compensated cirrhosis and ACLF[60]. Thus, regulation of the gut microbiome contributes to the development of cirrhosis[57]. Summarizing the epidemiological features of cirrhosis will be beneficial for providing clinical guidance and assistance. Globally, approximately 1 million people die annually from cirrhosis, the 11th most common cause of death, which is widely prevalent in low-, middle-, and high-income countries and is associated with high morbidity and mortality[1]. In 2019, an estimated 25% of global deaths from cirrhosis were alcohol-related[61]. Epidemiology-related recognition remains a hot topic and a frontier for future research. Various mouse models of liver fibrosis are often used in cirrhosis research, and the most commonly used are the toxic, biliary, and metabolic liver fibrosis models[62,63]. Recent studies on the mechanisms and pathways involved in hepatic fibrosis and cirrhosis have yielded promising results. The study of related pathways may shed light on the regulation of liver fibrosis and potential targets for therapy. For example, TGF-βpromotes liver fibrosis by activating Sma- and Mad-associated protein 3, intracellular IL-37 downregulates liver inflammation and fibrosis by interacting with Smad3[64], and curcumin inhibits liver fibrosis by activating autophagy to suppress TGF-β/Smad signaling, thereby inhibiting epithelial-mesenchymal transition and ECM production[65]. A study by Kong et al[66] showed that ferritin phagocytosis-mediated iron sagging in HSC is responsible for the antifibrotic effect induced by artesunate, which provides new clues for further pharmacological studies of artesunate. Zhang et al[67] found that melanoma differentiation-associated gene 5 effectively inhibits hepatic lipid accumulation and metabolic disorders by blocking the N-terminal dimerization of apoptosis signal-regulating kinase 1. Regardless of the etiology of the disease, obesity is an independent risk factor for hepatic decompensation[68]. Statistically, 20% of patients with NASH progress to cirrhosis and have an increased risk of developing HCC[69]. Dyslipidemia significantly contributes to cardiovascular-related mortality and morbidity in patients with NASH. Efluxifermin has been shown to remarkably reduce hepatic lipids and is usually safe and tolerated in patients with NASH (F1-F3) fibrosis, suggesting that efluxifermin may alter the progression of NASH[70]. However, further studies are required to investigate the specific mechanisms and effects. Numerous studies have been conducted on the potential role of EVs as biomarkers of liver diseases. EVs are significantly involved in intercellular communication by delivering different cargo and acting as vital cell-derived particles in the progression of liver injury and fibrosis, helping predict complications and mortality, detect early HCC, and predict the outcome of acute liver failure[71]. In the treatment of cirrhosis, EVs play a crucial role in anti-inflammatory mechanisms. For example, lipotoxic EVs mediate the adhesion of monocytes to liver sinusoidal endothelial cells mainly through an ITGβ1-dependent mechanism, and ITGβ1 exhibits an inflammatory inhibitory effect in a mouse model of NASH; however, further studies are needed to determine whether a similar therapeutic effect exists in human patients[62]. Gao et al[72] demonstrated that Src-homology 2 domain protein phosphatase 2 in HSC promotes the release of fibrotic EVs by inhibiting autophagy and Rho-associated protein kinase 1, and activating the mammalian target of the rapamycin pathway, which provides a new idea for a new target for liver fibrosis treatment. However, the exact mechanism of action of the autophagy-dependent EVs requires further investigation. A previous study[73] found that EVs released from a stem cell-like population derived from the human adult liver demonstrated antifibrotic and anti-inflammatory effects in an immunocompromised NASH mouse model, providing new clues for the treatment of cirrhosis. The role of bile acids in hepatic cirrhosis is receiving increasing attention. Bile acids can alter the microbiome and liver regeneration, and chronic cholestasis in PBC caused by the bile acid-affected microbiome can reversely lead to liver injury, creating a vicious cycle of chronic liver injury[46]. It was reported that cholesterol ameliorates bile acid toxicity in mouse models of NASH and fibrosis by inducing Nrf-2 and HIF-1α-dependent hepatocyte proliferation and liver regeneration[63]. One study[74] found that antagonizing TGR5 or inhibiting ERK1/2 and p38MAPK signaling may contribute to the prevention or reversal of liver fibrosis by reducing the hepatic concentrations of conjugated 12α-OH-bile acids. Salhab et al[75] found that the sodium+/taurocholate co-transporting polypeptide is a membrane transporter protein that affects the enterohepatic circulation of bile acids and that sodium+/taurocholate co-transporting polypeptide blockers can prevent the development of hepatic fibrosis. These findings provide further directions for specific targeted therapies for cirrhosis. The role of bile acids in the pathogenesis of cirrhosis is extremely complex and influenced by many mechanisms of interaction. Substantial research is required to determine their specific roles. ECM has also been one of the keywords of the outbreak in recent years. Liver fibrosis and cirrhosis are characterized by the abnormal deposition of ECM proteins. Alterations in ECM composition are critical for tissue repair, remodeling, and cellular responses to fibrosis[76]. A previous study[77] reported that surface-active vesicle-based ECM therapeutics can also treat liver fibrosis. Studying the interactions between fibroblasts, ECM, and inflammation may provide new avenues for identifying new therapeutic targets for cirrhosis[78]. To date, it is not fully understood how the ECM is dynamically remodeled during the development of cirrhosis, and whether ECM signaling has a predictive value for the prognosis of early cirrhosis still needs to be studied on a large scale[79].
Based on bibliometric analysis and literature visualization, the hotspots, evolutionary processes, and developmental trends of research on immunological factors in cirrhosis are presented visually, providing further insight into the current status and future perspectives compared to traditional reviews. Meanwhile, VOSviewer and CiteSpace have been used extensively for bibliometric analyses; therefore, our data analysis process was relatively objective and reliable. However, this study had some limitations. First, we retrieved publications and data from WoSCC; despite our efforts to obtain the most comprehensive literature, some articles were not included in the analysis, which may have led to bias. Second, owing to the time lag, some newly published high-quality papers did not receive sufficient citations and therefore could not be included in the analysis.
Immunological factors associated with cirrhosis have important research and application prospects. With the rapid increase in the number of studies, the study of immunological factors in cirrhosis has attracted great interest from researchers worldwide. The United States and China are the leading countries, and there is close cooperation between various countries and institutions. Current research hotspots regarding immunological factors in cirrhosis include fibrosis, cirrhosis, inflammation, liver fibrosis, expression, HCC, activation, PBC, disease, and HSCs. Immune dysfunction, gut microbiota, epidemiology, mouse model, pathway, obesity, EVs, bile acids, and the ECM are currently at the frontier of research on immunological factors in cirrhosis. It is notable that in addition to focusing on basic research, we should also pay attention to the translation and application of the research results.
Cirrhosis is deemed a systemic disease associated with the immune system resulting from a persistent liver injury leading to liver fibrosis. Immunological factors play important regulatory roles in the development and progression of cirrhosis.
Bibliometrics is one of the most used methods for the systematic evaluation of a field of study. To date, no bibliometric studies have been conducted on the role of immunological factors in cirrhosis. A statistical analysis conducted by a bibliometric method may provide a more comprehensive understanding and assessment of the hot spots and trends in research on immunological factors in cirrhosis disease.
To provide a comprehensive overview of the knowledge structure and research hotspots of immunological factors in cirrhosis, which can help scholars quickly understand the research status and provide new ideas and directions for studying immunological factors in cirrhosis.
We retrieved publications related to immunological factors in cirrhosis between 2003 to 2022 from the Web of Science Core Collection (WoSCC) database on December 7, 2022. The search strategy was TS = ((Liver Cirrhosis OR hepatic cirrhosis OR liver fibrosis) AND (Immunologic* Factor* OR Immune Factor* OR Immunomodulator* OR Biological Response Modifier* OR Biomodulator*)). Only original articles and reviews were included. A total of 2873 publications were analyzed using indicators of publication and citation metrics, countries, institutes, authors, journals, references, and keywords by CiteSpace and VOSviewer.
A total of 5104 authors from 1173 institutions across 51 countries published 2873 papers on cirrhosis and immunological factors in 281 journals. In this article, we explored and analyzed research in this field according to country, journal, author, and other relevant factors. Current research hotspots in this field include fibrosis, cirrhosis, inflammation, liver fibrosis, expression, hepatocellular carcinoma, activation, primary biliary cirrhosis, disease, and hepatic stellate cells. Burst keywords (e.g., epidemiology, gut microbiota, and pathways) represent research frontiers that have attracted the interest of researchers in recent years.
We used bibliometrics, a relatively new literature research method, to analyze the knowledge structure and hotspots of research in the field, providing new ideas for promoting scientific research and clinical applications.
Burst keywords, including immune dysfunction, gut microbiota, epidemiology, mouse models, pathways, obesity, extracellular vesicles, bile acids, and the extracellular matrix, represent the current frontiers of research on immunological factors in cirrhosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Navarro-Alvarez N, Mexico; Paparoupa M, Germany S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 453] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 2. | Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 461] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 3. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 4. | Fortea JI, Crespo J, Puente Á. Cirrhosis, a Global and Challenging Disease. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 674] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Dong Y, Wu X, Wang X, Niu J. Identification of Immune Microenvironment Changes and the Expression of Immune-Related Genes in Liver Cirrhosis. Front Immunol. 2022;13:918445. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 7. | Hou J, Su H, Kuang X, Qin W, Liu K, Pan K, Zhang B, Yang S, Peng X, Nie X, Hua Q. Knowledge Domains and Emerging Trends of Osteoblasts-Osteoclasts in Bone Disease From 2002 to 2021: A Bibliometrics Analysis and Visualization Study. Front Endocrinol (Lausanne). 2022;13:922070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Devos P, Menard J. Bibliometric analysis of research relating to hypertension reported over the period 1997-2016. J Hypertens. 2019;37:2116-2122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Zheng MQ, Li XX, Xu R, Liu S, Rui ZY, Guo ZY, Chen D. Bibliometric analysis of tuberculosis molecular epidemiology based on CiteSpace. Front Public Health. 2022;10:1040176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 10. | Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A Bibliometric Analysis of Top-Cited Journal Articles in Obstetrics and Gynecology. JAMA Netw Open. 2019;2:e1918007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Barboza-Palomino M, Salas G, Vega-Arce M, Caycho-Rodríguez T, Ventura-León J, Flores-Kanter PE, Salas-Blas E, Landa-Barzola M, López-López W. Thirty Years of Psicothema: A Bibliometric Analysis (1989-2018). Psicothema. 2020;32:459-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Gantenbein L, Arora P, Navarini A, Brandt O, Mueller SM. Global publication productivity in dermatology: a bibliometric description of the past and estimation of the future. J Eur Acad Dermatol Venereol. 2021;35:1424-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Tárnok A. Bibliometric news and more about signal transduction and disease. Cytometry A. 2021;99:764-765. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Huang YJ, Huang CJ. Construction of a 5 immune-related lncRNA-based prognostic model of NSCLC via bioinformatics. Medicine (Baltimore). 2021;100:e27222. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Xu X, Wang Y, Li Y, Zhang B, Song Q. The Future Landscape of Macrophage Research in Cardiovascular Disease: A Bibliometric Analysis. Curr Probl Cardiol. 2022;47:101311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 16. | Liu B, He X, Wang Y, Huang JW, Zheng YB, Li Y, Lu LG. Bibliometric Analysis of γδ T Cells as Immune Regulators in Cancer Prognosis. Front Immunol. 2022;13:874640. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Liu S, Sun YP, Gao XL, Sui Y. Knowledge domain and emerging trends in Alzheimer's disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. 2019;14:1643-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Lin J, Ling F, Huang P, Chen M, Song M, Lu K, Wang W. The Development of GABAergic Network in Depression in Recent 17 Years: A Visual Analysis Based on CiteSpace and VOSviewer. Front Psychiatry. 2022;13:874137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Chen P, Zhong C, Jin S, Zhang Y, Li Y, Xia Q, Cheng J, Fan X, Lin H. Global Trends in Research of Lipid Metabolism in T lymphocytes From 1985 to 2022: A Bibliometric Analysis. Front Immunol. 2022;13:884030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 707] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 21. | Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 456] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 22. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1596] [Article Influence: 228.0] [Reference Citation Analysis (0)] |
| 23. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 736] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 24. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3150] [Article Influence: 450.0] [Reference Citation Analysis (0)] |
| 25. | Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, Zhang L. Knowledge Mapping of Exosomes in Autoimmune Diseases: A Bibliometric Analysis (2002-2021). Front Immunol. 2022;13:939433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Tanaka H, Leung PS, Kenny TP, Gershwin ME, Bowlus CL. Immunological orchestration of liver fibrosis. Clin Rev Allergy Immunol. 2012;43:220-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 29. | Ma HD, Wang YH, Chang C, Gershwin ME, Lian ZX. The intestinal microbiota and microenvironment in liver. Autoimmun Rev. 2015;14:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 812] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 31. | Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 32. | Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Sahin H, Trautwein C, Wasmuth HE. Functional role of chemokines in liver disease models. Nat Rev Gastroenterol Hepatol. 2010;7:682-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 35. | Frissen M, Liao L, Schneider KM, Djudjaj S, Haybaeck J, Wree A, Rolle-Kampczyk U, von Bergen M, Latz E, Boor P, Trautwein C. Bidirectional Role of NLRP3 During Acute and Chronic Cholestatic Liver Injury. Hepatology. 2021;73:1836-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Bernsmeier C, van der Merwe S, Périanin A. Innate immune cells in cirrhosis. J Hepatol. 2020;73:186-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 37. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 472] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 38. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 190] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 39. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 689] [Article Influence: 229.7] [Reference Citation Analysis (0)] |
| 40. | Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 237] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 41. | Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7:267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 42. | Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, Acevedo J, Duran-Güell M, Nuñez L, Costa M, Torres M, Horrillo R, Ruiz-Del-Árbol L, Villanueva C, Prado V, Arteaga M, Trebicka J, Angeli P, Merli M, Alessandria C, Aagaard NK, Soriano G, Durand F, Gerbes A, Gustot T, Welzel TM, Salerno F, Bañares R, Vargas V, Albillos A, Silva A, Morales-Ruiz M, Carlos García-Pagán J, Pavesi M, Jalan R, Bernardi M, Moreau R, Páez A, Arroyo V. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology. 2019;157:149-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 146] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 43. | Garrido A, Djouder N. Cirrhosis: A Questioned Risk Factor for Hepatocellular Carcinoma. Trends Cancer. 2021;7:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Baglieri J, Brenner DA, Kisseleva T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 45. | Gulamhusein AF, Hirschfield GM. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020;17:93-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 46. | Tanaka A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol. 2021;27:1-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, Goria O, Potier P, Minello A, Silvain C, Abergel A, Debette-Gratien M, Larrey D, Roux O, Bronowicki JP, Boursier J, de Ledinghen V, Heurgue-Berlot A, Nguyen-Khac E, Zoulim F, Ollivier-Hourmand I, Zarski JP, Nkontchou G, Lemoinne S, Humbert L, Rainteau D, Lefèvre G, de Chaisemartin L, Chollet-Martin S, Gaouar F, Admane FH, Simon T, Poupon R. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171-2181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 314] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 48. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 551] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 49. | Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5:306-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Mayo MJ. Mechanisms and molecules: What are the treatment targets for primary biliary cholangitis? Hepatology. 2022;76:518-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 813] [Article Influence: 116.1] [Reference Citation Analysis (0)] |
| 52. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 138] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 53. | Lurje I, Hammerich L, Tacke F. Dendritic Cell and T Cell Crosstalk in Liver Fibrogenesis and Hepatocarcinogenesis: Implications for Prevention and Therapy of Liver Cancer. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 112] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 55. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 313] [Article Influence: 78.3] [Reference Citation Analysis (2)] |
| 56. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 57. | Lee NY, Suk KT. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int J Mol Sci. 2020;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70:982-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 59. | Campana L, Esser H, Huch M, Forbes S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. 2021;22:608-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 60. | Trebicka J, Bork P, Krag A, Arumugam M. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat Rev Gastroenterol Hepatol. 2021;18:167-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 61. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 64] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 62. | Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H, Gao Y, Revzin A, Ibrahim SH. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 63. | Kaminsky-Kolesnikov Y, Rauchbach E, Abu-Halaka D, Hahn M, García-Ruiz C, Fernandez-Checa JC, Madar Z, Tirosh O. Cholesterol Induces Nrf-2- and HIF-1α-Dependent Hepatocyte Proliferation and Liver Regeneration to Ameliorate Bile Acid Toxicity in Mouse Models of NASH and Fibrosis. Oxid Med Cell Longev. 2020;2020:5393761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Mountford S, Effenberger M, Noll-Puchta H, Griessmair L, Ringleb A, Haas S, Denk G, Reiter FP, Mayr D, Dinarello CA, Tilg H, Bufler P. Modulation of Liver Inflammation and Fibrosis by Interleukin-37. Front Immunol. 2021;12:603649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Kong D, Zhang Z, Chen L, Huang W, Zhang F, Wang L, Wang Y, Cao P, Zheng S. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020;36:101600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 66. | Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043-2053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 67. | Zhang X, Yang H, Zeng S, Tian S, Hu S, Yang L, Ma T, Liu Z, Wan J, Zhong Y, Li H. Melanoma differentiation-Associated gene 5 protects against NASH in mice. Hepatology. 2022;75:924-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | El-Sherif O, Armstrong MJ. Peculiarities of Cirrhosis due to Nonalcoholic Steatohepatitis (NASH). Semin Liver Dis. 2020;40:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 696] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 70. | Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA, Hu C, Fong E, de Temple B, Tillman EJ, Rolph TP, Cheng A, Yale K. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 71. | Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: A clinician's point of view. J Hepatol. 2020;73:1507-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 72. | Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Cooper SA, Cao S, Shah VH, Kostallari E. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 73. | Bruno S, Pasquino C, Herrera Sanchez MB, Tapparo M, Figliolini F, Grange C, Chiabotto G, Cedrino M, Deregibus MC, Tetta C, Camussi G. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-alcoholic Steatohepatitis. Mol Ther. 2020;28:479-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 74. | Xie G, Jiang R, Wang X, Liu P, Zhao A, Wu Y, Huang F, Liu Z, Rajani C, Zheng X, Qiu J, Zhang X, Zhao S, Bian H, Gao X, Sun B, Jia W. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine. 2021;66:103290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 75. | Salhab A, Amer J, Lu Y, Safadi R. Sodium(+)/taurocholate cotransporting polypeptide as target therapy for liver fibrosis. Gut. 2022;71:1373-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | de Castro Brás LE, Frangogiannis NG. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020;91-92:176-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 77. | Geervliet E, Moreno S, Baiamonte L, Booijink R, Boye S, Wang P, Voit B, Lederer A, Appelhans D, Bansal R. Matrix metalloproteinase-1 decorated polymersomes, a surface-active extracellular matrix therapeutic, potentiates collagen degradation and attenuates early liver fibrosis. J Control Release. 2021;332:594-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 78. | Moretti L, Stalfort J, Barker TH, Abebayehu D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J Biol Chem. 2022;298:101530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 79. | Wu Y, Cao Y, Xu K, Zhu Y, Qiao Y, Wu Y, Chen J, Li C, Zeng R, Ge G. Dynamically remodeled hepatic extracellular matrix predicts prognosis of early-stage cirrhosis. Cell Death Dis. 2021;12:163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |