Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3843
Peer-review started: April 10, 2023
First decision: April 28, 2023
Revised: May 13, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: June 28, 2023
Research exploring the influence of healthier lifestyle modification (LSM) on the risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB) is limited.
To emulate a target trial to determine the effect of LSM on HCC incidence and mortality among patients with CHB by large-scale population-based observational data.
Among the patients with CHB enrolled in the Korean National Health Insurance Service between January 1, 2009, and December 31, 2017, those aged ≥ 20 years who drank alcohol, smoked cigarettes, and were sedentary were analyzed. Exposure included at least one LSM, including alcohol abstinence, smoking cessation, and regular exercise. The primary outcome was HCC development, and the secondary outcome was liver-related mortality. We used 2:1 propensity score matching to account for covariates.
With 48766 patients in the LSM group and 103560 in the control group, the adjusted hazard ratio (HR) for incident HCC and liver-related mortality was 0.92 [95% confidence interval (CI): 0.87-0.96] and 0.92 (95%CI: 0.86-0.99) in the LSM group, respectively, compared with the control group. Among the LSM group, the adjusted HR (95%CI) for incident HCC was 0.84 (0.76-0.94), 0.87 (0.81-0.94), and 1.08 (1.00-1.16) for alcohol abstinence, smoking cessation, and regular exercise, respectively. The adjusted HR (95%CI) for liver-related mortality was 0.92 (0.80-1.06), 0.81 (0.72-0.91), and 1.15 (1.04-1.27) for alcohol abstinence, smoking cessation, and regular exercise, respectively.
LSM lowered the risk of HCC and mortality in patients with CHB. Thus, active LSM, particularly alcohol abstinence and smoking cessation, should be encouraged in patients with CHB.
Core Tip: Unhealthy behaviors, including smoking, sedentary lifestyle, or alcohol drinking, are known to have adverse effect on the outcome among chronic hepatitis B (CHB) patients. However, there are limited data on the effect of healthier lifestyle modification (LSM), including quitting smoking, regular exercise, and quitting drinking, on the risk of hepatocellular carcinoma (HCC) in CHB patients. In this first hypothetical randomized trial, we demonstrated that the LSM lowers the risk of developing HCC by using the nationwide database. The findings of this study highlight the necessity for active counseling and therapeutic intervention for LSM in CHB patients.
- Citation: Park Y, Kang D, Sinn DH, Kim H, Hong YS, Cho J, Gwak GY. Effect of lifestyle modification on hepatocellular carcinoma incidence and mortality among patients with chronic hepatitis B. World J Gastroenterol 2023; 29(24): 3843-3854
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3843.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3843
Chronic hepatitis B virus (HBV) infection is the leading cause of hepatocellular carcinoma (HCC) and accounts for 56% of all cases[1,2]. Despite the introduction of neonatal immunization to prevent vertical transmission and antiviral medications with high genetic barrier, the risk of HCC remains high[3]. Thus, ongoing efforts are being made to further decrease the global health burden of chronic hepatitis B (CHB) with modifiable risk factors.
Mounting evidence indicates that not only viral factors but also host factors, such as alcohol consumption, smoking, and obesity, and diet influence the prognosis of patients with CHB[4-7]. Alcohol drinking was associated with increased risk of HCC[8] and mortality[9]. A meta-analysis reported that smoking elevated the risk of HCC by 1.44 times through an additive interaction[10], and a recent prospective cohort study on patients with diabetes and CHB showed pack per year dose responsiveness[7]. Insulin resistance[11] and central obesity[12] increase the incidence of liver cancer in patients with CHB. In contrast, increased physical activity can lower the incidence of liver cancer[13]. Correspondingly, few studies have evaluated whether the modification on behavioral risk factors reduces the incidence of HCC, although studies have demonstrated that the risk of developing HCC increases with unhealthy behavior[14]. A meta-analysis attempted to confirm whether alcohol abstinence prevents HCC; however, reliable results were not obtained owing to the lack of quality studies[14]. Evidence from observational studies and meta-analyses suggests that coffee consumption can reduce the incidence of HCC including in patients with CHB[15,16]. Furthermore, several studies have de-monstrated that food-derived components may have a positive impact on the progression of liver fibrosis[17,18] and the treatment of HCC[19-21]. Randomized controlled trials (RCTs) are required to confirm the benefits of modifying behavioral risk factors for HCC in patients with CHB. However, no RCT on patients with CHB has evaluated the impact of lifestyle modifications (LSM) on the incidence of HCC. This might be because RCTs require enormous resources and time, including medical care, patient participation, human resources, and time to observe events.
An emulated target trial using observational data can help avoid methodological pitfalls in observational studies[22]. It can yield the same estimated effects as that of an RCT if the emulation is successful[23]. HBV infection is the most common cause of HCC in South Korea[24]. South Korea has government-mandated universal health insurance coverage for the entire population[25]. The Korean National Health Insurance Service (K-NHIS) claims database includes information on disease and treatment, as well as health behaviors. Thus, we conducted an emulation trial to determine the effect of LSM on HCC incidence and mortality among patients with CHB using nationwide registry data.
We performed a sequential trial emulation study using the K-NHIS database. The K-NHIS database represents the entire South Korean population. The K-NHIS claims database contains information on demographics, medical treatment, procedures, prescription drugs, diagnostic codes, and hospital use. Diagnoses in the K-NHIS database were based on the International Classification of Diseases, 10th revision (ICD-10). In addition, the K-NHIS claims database includes data from the National Health Screening Examination (NHSE), which is a standardized health screening program provided to all insured persons every 2 years[26]. The NHSE includes a self-administered questionnaire on medical history and health behaviors; including smoking, drinking, and physical activity; anthropometric measurements; and laboratory tests. The participation rate of the NHSE in the target population is approximately 76%. The use of the K-NHIS database was approved by the NHIS Review Committee (protocol number: NHIS-2021-1-575). All patients received an anonymous identification code in the K-NHIS data to protect the patients' information and identity.
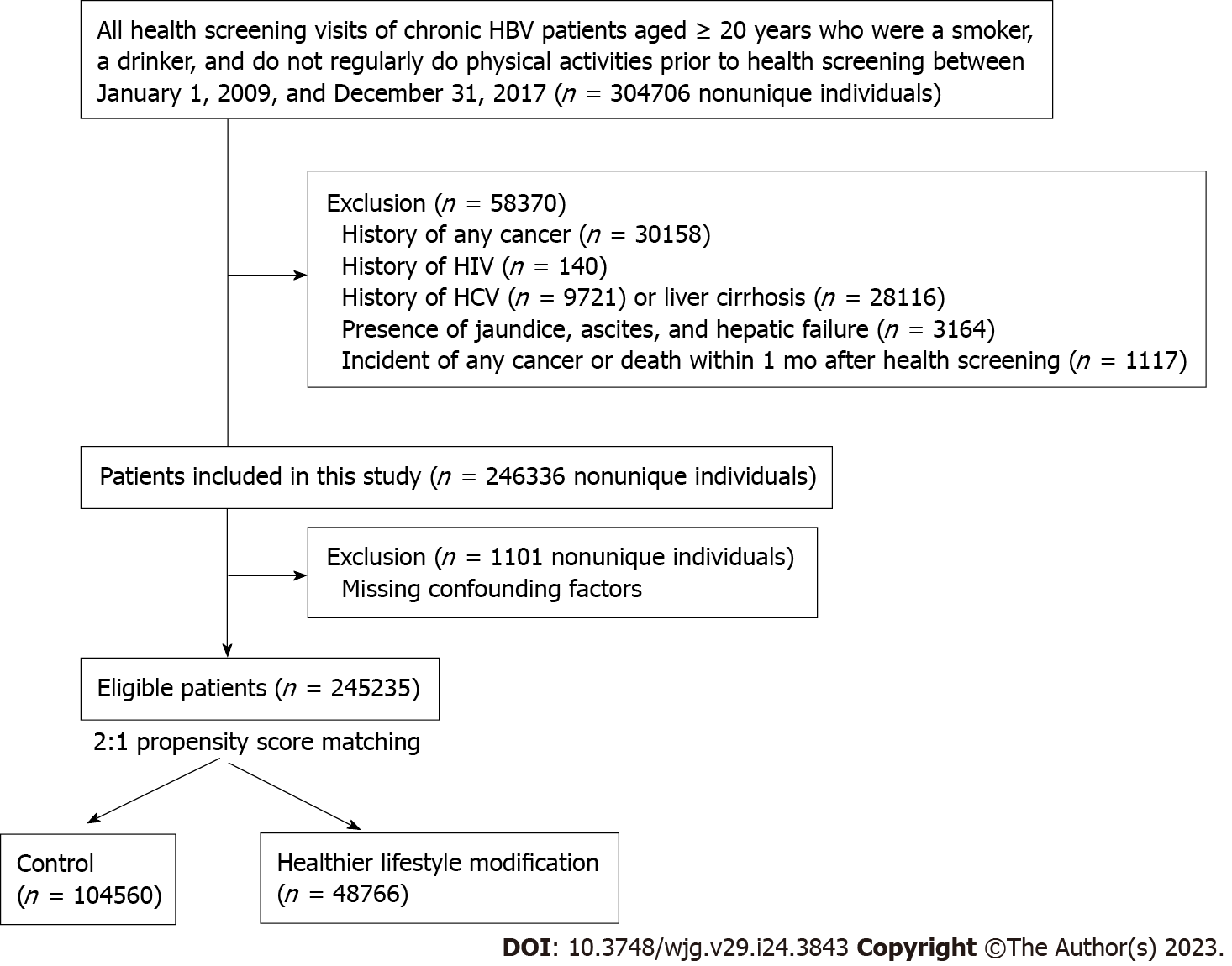
This study included participants aged ≥ 20 years with CHB, which was defined as the presence of HBV (B18.0, B18.1, B18.10, B18.18, or Z22.5) codes in any claim or presented in a death certificate during the study period. We restricted participants to those who were drinking, smoking, and did not exercise regularly during the health screening exam between January 1, 2009, and December 31, 2017, as our study aimed to evaluate the benefits of LSM. We then selected participants who had at least one additional health screening examination to evaluate changes in lifestyle (baseline). We excluded participants with a history of cancer, human immunodeficiency virus (HIV) infection (ICD-10 codes: B20, B21, B22, and B24), hepatitis C virus (HCV) infection (ICD 10 code: B18.2), liver cirrhosis (ICD-10 codes: K70.2, K70.3, K71.7, K76.1, and K74), jaundice (ICD-10 code: R17), ascites (ICD-10 code: R18), and hepatic failure (ICD-10 code: K720). Furthermore, we excluded participants who developed cancer or died within the first month of follow-up to minimize potential reverse causality. We also excluded those with missing data for matching variables. The Institutional Review Board of Samsung Medical Center approved the study and waived the requirement for informed consent because the K-NHIS data were de-identified.
The main exposure was LSM including alcohol abstinence, smoking cessation, and regular exercise. The NHSE used standardized questionnaires to obtain information on alcohol drinking, smoking, and exercise. For the information on smoking status, participants were asked if they had ever smoked at least 100 cigarettes in their lifetime, based on the World Health Organization definition. Ever smokers were then asked regarding the duration of smoking and the mean number of cigarettes smoked per day. For drinking, participants were asked for the frequency (number of days per week) and quantity (amount of standard unit per occasion) of alcohol consumption. A standard unit was defined as a specialized cup for each type of alcohol such as beer, wine, Korean traditional alcohol (soju), or whisky. One standard unit contains roughly 8 g of ethanol in Korea, although different drinks can have very different alcohol content. The weekly amount of alcohol consumption was calculated by multiplying these two values. Alcohol intake was categorized into none, moderate (< 40 g/d in women and < 60 g/d in men), and heavy (≥ 40 g/d in women and ≥ 60 g/d in men). The exercise questionnaire used a 7-d recall method. The questionnaire was similar to the International Physical Activity Questionnaire (IPAQ)-Short form, which was modified by the Korean NHIS (see Supplementary Figure 1). The questionnaire consisted of the following three questions: (1) How many days in the past week did you engage in vigorous activities that made you breathe much harder than normal for at least 20 min per day (e.g., running, aerobics, fast biking, climbing, etc.)? (2) How many days in the past week did you engage in moderate activities that made you breathe somewhat harder than normal for at least 30 min per day (e.g., fast walking, doubles tennis, riding a bicycle at a normal speed, mopping, etc.)? and (3) How many days in the past week did you engage in light activities, such as walking for at least 30 min per day, adding up to a total of at least 30 min per day (e.g., walking to and from work or for leisure, light household chores, etc.)? This questionnaire has been widely used in previous studies. The main exposure was regular physical activity, which was defined as vigorous physical activity for ≥ 3 d per week (at least 60 min per week) or moderate physical activity for ≥ 5 d per week (at least 150 min per week).
The primary endpoint was the development of HCC. HCC was defined as the presence of a cancer-specific insurance claim code (V193 code) with the C22.0 code, which is ICD-10 code for HCC. The secondary endpoint was liver-related mortality. Vital status and cause of death were obtained from death certificates collected by the Statistics Korea at the Ministry of Strategy and Finance of South Korea[27]. We considered it a liver-related death if the patients had liver cancer (C22) or liver disease (B15–B19, K70–K75) codes for the cause of death. We also included the development of all types of cancer, extrahepatic cancer, and all-cause mortality as the endpoints.
We included age, sex, body mass index (BMI), liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT)], comorbidities, and antiviral medications at baseline as covariates. Daily alcohol consumption, drinking status, smoking status, and level of physical activity at the time of inclusion were also analyzed.
We emulated a pragmatic sequence of trials by aligning the eligibility window, group assignment, and start of follow-up between study arms[28]. We identified patients with CHB who met the eligibility criteria on the day of health-screening visits to emulate a trial of the effectiveness of LSM on clinical outcomes in patients with CHB. We repeated the enrollment process for all available health screening visits. Each participant could contribute as an eligible individual to as many trials as possible. The emulation of sequential trials is a valid and efficient procedure if participants meet the eligibility criteria at several time points[29]. The participants were then reclassified into two groups according to LSM at the visit. In all trials, participants were followed from baseline until the development of HCC, death, or December 2019, whichever occurred first.
Furthermore, propensity score (PS) matching was performed to minimize the potential impact of confounders on outcomes. We conducted multivariable logistic regression using the following covariates: Age, BMI, AST, ALT, GGT, presence of hypertension, diabetes, myocardial infarction, congestive heart failure, cerebrovascular accident, whether the participant took antiviral medications at the current health screening visit, drinking status (moderate or heavy), smoking status, daily alcohol consumption, and physical activity status (not at all or a little) at the previous health screening visit to estimate the PS for the LSM group. Furthermore, if a patient had health screenings at different time points, we followed them from the date of their first screening, and then the time since the previous exam was included in the matching variables. Matching was performed using a greedy algorithm (caliper = 0.1). The control group was matched 2:1 with the LSM group in each trial. The covariate variables were updated at the start of each trial in the analysis. The covariates were updated over time following the sequential target emulate trial methods. This methodological strategy aligned the assessment of eligibility criteria, treatment assignment, and starting of follow-up, thus removing any immortal-time bias related to the delay between the health screening visit and adoption of healthy behaviors on clinical outcomes. We pooled data from all trials into a single model and included the day of the trial’s baseline in the analysis.
The primary analysis was intention-to-treat analysis. Cumulative incidence of each outcome was estimated using the Kaplan-Meier method, and log rank tests were used to evaluate differences between the groups. We calculated hazard ratios (HR) with 95% confidence intervals (CI) for incidence of clinical outcome using a Cox regression model. Covariates with SMD > 0.1, which can provide evidence of an imbalance between matched groups, were adjusted for survival analysis.
We examined the proportional hazards assumption using plots of the log(-log) survival function and Schoenfeld residuals. All P values were two-sided, and a P value < 0.05 was considered as significant. Analyses were performed using of SAS® Visual Analytics (SAS Institute Inc., United States) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Of the 304706 potential person-trials, we excluded participants with a history of any cancer (n = 30158), HIV (n = 140), HCV (n = 9721) infection, or liver cirrhosis (n = 28116). We also excluded participants who had jaundice, ascites, or hepatic failure (n = 3164) and 1117 participants who developed cancer or died within the first month of follow-up. Thus, 246336 person-trials met the eligibility criteria; however, 1101 person-trials were excluded because of missing data on confounding factors. Therefore, the final sample size included 245235 person-trials. Among them, we selected two person-trials for the control group per LSM person-trial. Thus, we obtained a control group of 104560 person-trials matched with 48766 person-trials from the LSM group (Figure 1). In the LSM group, 33.7%, 29.6%, and 16.9% of participants belonged to the quitting smoking (QS), regular exercise (RE), and quitting drinking (QD) groups, respectively. In addition, 1.5% of the participants belonged to the QD + QS + RE group.
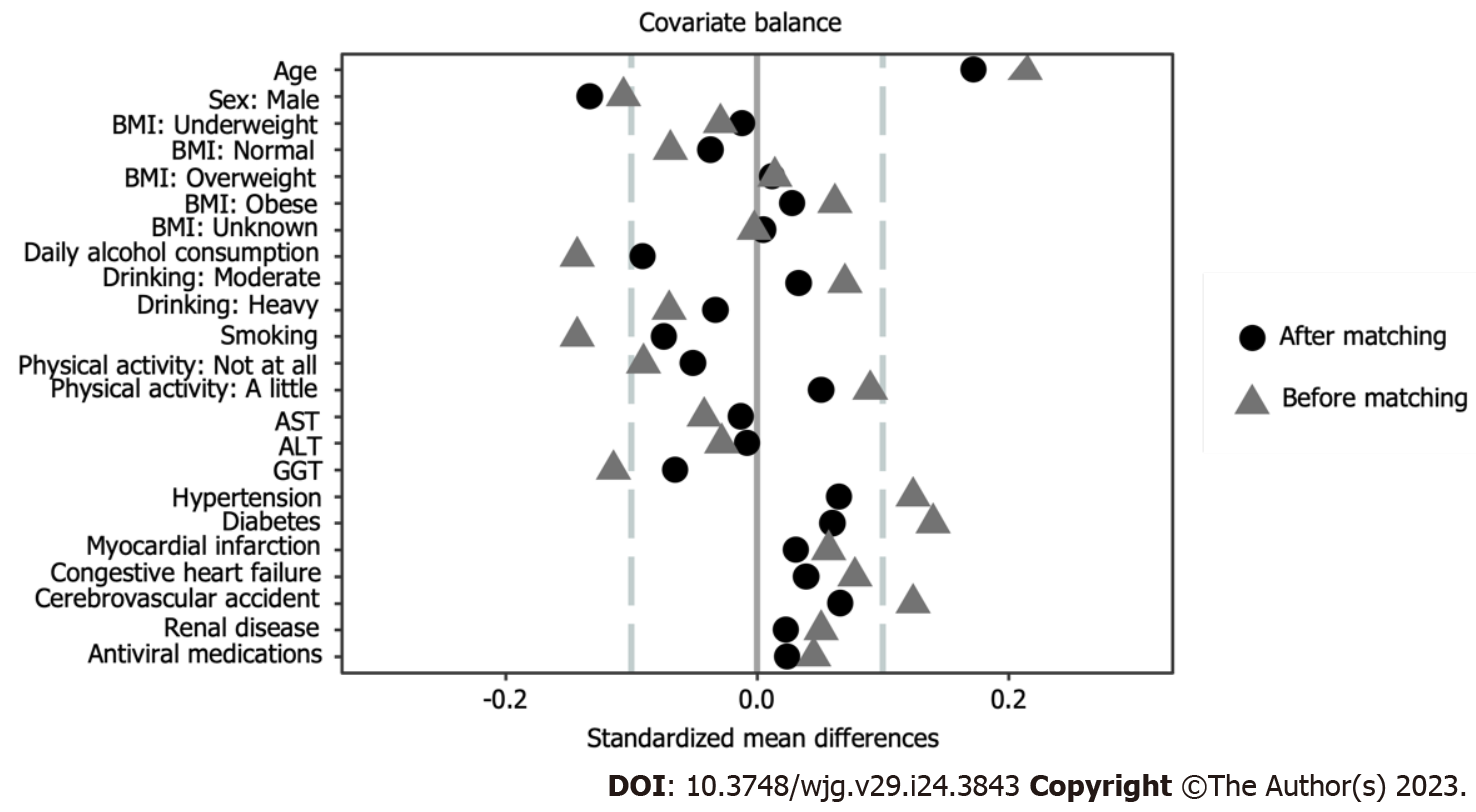
The mean age of the study participants was 50 years, and 95% of them were men (Table 1). All SMDs of the difference between the control and LSM groups were less than 0.1, except for age and sex (Figure 2). The LSM group was more likely to be older and female compared with the control group.
| Control (n = 104560) | LSM (n = 48766) | SMD | |
| Age, yr | 50.1 (7.4) | 51.4 (8.0) | 0.122 |
| Sex | -0.133 | ||
| Male | 100642 (96.3) | 45496 (93.3) | |
| Female | 3918 (3.7) | 3270 (6.7) | |
| BMI | |||
| Underweight (< 18.5 kg/m2) | 2017 (1.9) | 860 (1.8) | 0.028 |
| Normal (18.5-23 kg/m2) | 34098 (32.6) | 15065 (30.9) | -0.012 |
| Obesity (23-25 kg/m2) | 27019 (25.8) | 12856 (26.4) | -0.037 |
| Overweight (> 25 kg/m2) | 41413 (39.6) | 19976 (41.0) | 0.012 |
| Unknown | 13 (0.0) 0.012 | 9 (0.0) | 0.005 |
| Daily alcohol consumption1, g/d | 28.8 (27.1) | 26.3 (27.5) | -0.064 |
| Drinking status1 | |||
| Moderate | 92707 (88.7) | 43734 (89.7) | 0.097 |
| Heavy | 11853 (11.3) | 5032 (10.3) | -0.033 |
| Smoking1, pack-year | 19.1 (11.8) | 18.2 (12.9) | -0.052 |
| Physical activity status1 | 0.051 | ||
| Not at all | 56184 (53.7) | 24967 (51.2) | |
| A little | 48376 (46.3) | 23799 (48.8) | |
| AST, U/L | 38.0 (49.6) | 37.3 (45.8) | -0.009 |
| ALT, U/L | 40.8 (60.8) | 40.3 (59.6) | -0.006 |
| GGT, U/L | 76.6 (91.8) | 69.5 (94.2) | -0.046 |
| Hypertension (%) | 27297 (26.1) | 14145 (29.0) | 0.065 |
| Diabetes (%) | 22475 (21.5) | 11712 (24.0) | 0.06 |
| Myocardial infarction (%) | 838 (0.8) | 537 (1.1) | 0.031 |
| Congestive heart failure (%) | 2142 (2.0) | 1288 (2.6) | 0.039 |
| Cerebrovascular accident (%) | 5150 (4.9) | 3145 (6.4) | 0.066 |
| Renal disease (%) | 867 (0.8) | 515 (1.1) | 0.023 |
| Antiviral medications (%) | 896 (0.9) | 533 (1.1) | -0.024 |
During follow-up, 14401 participants developed cancer, and 50.8% of the incident cancers were HCC. The fully adjusted HR for incident HCC comparing LSM group to the control group was 0.92 (95%CI: 0.87-0.96). The association between LSM and HCC was consistent in all subgroups analyzed, except for sex (Table 2). The QD (HR = 0.84, 95%CI: 0.76-0.94) and QS groups (HR = 0.87, 95%CI: 0.81-0.94) showed a beneficial effect on reducing incident HCC compared with the control group when we subcategorized LSM. The fully adjusted HR for incident all cancer and extrahepatic cancer comparing the LSM group to the control group was 0.93 (95%CI: 0.90-0.97) and 0.96 (95%CI: 0.91-1.01), respectively (Table 2).
| No. of case (100 persons year) | HR (95%CI) | |
| Hepatocellular carcinoma | ||
| Control (n = 104560) | 5031 (0.8) | Reference |
| LSM1 (n = 48766) | 2296 (0.8) | 0.92 (0.87-0.96) |
| QD (n = 8224) | 371 (0.7) | 0.84 (0.76-0.94) |
| QS (n = 16423) | 695 (0.7) | 0.87 (0.81-0.94) |
| RE (n = 14457) | 799 (0.9) | 1.08 (1.00-1.16) |
| QD + QS + RE (n = 686) | 31 (0.7) | 0.79 (0.55-1.12) |
| All cancer | ||
| Control (n = 104560) | 9691 (1.6) | Reference |
| LSM (n = 48766) | 4710 (1.6) | 0.93 (0.90-0.97) |
| QD (n = 8224) | 826 (1.7) | 0.92 (0.86-0.99) |
| QS (n = 16423) | 1411 (1.5) | 0.89 (0.84-0.94) |
| RE (n = 14457) | 1506 (1.8) | 1.04 (0.99-1.10) |
| QD + QS + RE (n = 686) | 70 (1.7) | 0.85 (0.67-1.07) |
| Extrahepatic cancer | ||
| Control (n = 104560) | 4660 (0.8) | Reference |
| LSM (n = 48766) | 2414 (0.8) | 0.96 (0.91-1.01) |
| QD (n = 8224) | 455 (0.9) | 1.01 (0.92-1.11) |
| QS (n = 16423) | 716 (0.8) | 0.91 (0.84-0.99) |
| RE (n = 14457) | 707 (0.8) | 1.00 (0.93-1.09) |
| QD + QS + RE (n = 686) | 39 (0.9) | 0.91 (0.67-1.25) |
During the follow-up period, 6833 participants died, and 52.8% were liver-related death. The LSM group had a significantly lower risk of liver-related death compared with the control group over the entire period. The fully adjusted HR for all-cause mortality, when comparing the LSM group with the control group, was 0.94 (95%CI: 0.90-0.99) (Table 3). The fully adjusted HR for liver-related mortality, when comparing the LSM group with the control group, was 0.92 (95%CI: 0.86-0.99) (Table 3).
| No. of case (100 persons year) | HR (95%CI) | |
| Liver-related mortality | ||
| Control (n = 104560) | 2458 (0.4) | Reference |
| LSM1 (n = 48766) | 1150 (0.4) | 0.92 (0.86-0.99) |
| QD (n = 8224) | 203 (0.4) | 0.92 (0.80-1.06) |
| QS (n = 16423) | 319 (0.3) | 0.81 (0.72-0.91) |
| RE (n = 14457) | 422 (0.5) | 1.15 (1.04-1.27) |
| QD + QS + RE (n = 686) | 17 (0.4) | 0.85 (0.53-1.37) |
| All-cause mortality | ||
| Control (n = 104560) | 4539 (0.7) | Reference |
| LSM (n = 48766) | 2294 (0.7) | 0.94 (0.90-0.99) |
| QD (n = 8224) | 451 (0.9) | 1.04 (0.94-1.15) |
| QS (n = 16423) | 588 (0.6) | 0.78 (0.72-0.85) |
| RE (n = 14457) | 772 (0.9) | 1.11 (1.03-1.20) |
| QD + QS + RE (n = 686) | 38 (0.9) | 0.91 (0.66-1.25) |
This study demonstrated that LSM is associated with a reduced incidence of HCC and liver-related mortality in patients with CHB. Specifically, alcohol abstinence and smoking cessation were associated with a reduced incidence of HCC, while smoking cessation was associated with reduced liver-related and all-cause mortality.
We included patients with CHB who drank, smoked, and did not exercise regularly. As the risk of developing HCC increases with the presence of more behavioral risk factors[30], the participants of this study are expected to have the poorest prognosis. We found that the risk of HCC was reduced by 8% with the adoption of one LSM. Specifically, the risk was reduced by 16%, 13%, and -8% with alcohol abstinence, smoking cessation, and regular exercise, respectively. Risk was reduced by 21% when all LSM components were adopted. These findings strongly suggest that patients with CHB should be encouraged to adopt healthier lifestyles, particularly alcohol abstinence and smoking cessation.
To date, little is known regarding the effects of LSM on HCC and liver-related mortality in patients with CHB. Alcohol consumption increases the incidence of HCC[31,32]. A meta-analysis on the effect of alcohol abstinence in patients with CHB predicted that limiting alcohol consumption would have a protective effect against the development of HCC even though quality research has not been conducted[14]. Abstinence is believed to counteract some effects of alcohol. Drinking upregulates cytochrome P450 2E1 (CYP2E1), which promotes the generation of reactive oxygen species and electrolyte leakage in the mitochondrial respiratory chain[33]. After 3 d of abstinence, the levels of CYP2E1 have been shown to decrease rapidly, corroborating our findings[34].
Smoking is another important risk factor for HCC development from the host side. In 2010, the Surgeon General’s report expanded the list of smoking-related cancers to include HCC[35]. Few studies have demonstrated the effects of smoking cessation, although smoking is widely acknowledged as a risk factor for HCC. Two prospective observational studies examined the incidence of HCC, ALT elevation, and fibrosis score; however, no statistically significant results were obtained whether smoking cessation had a positive effect on the liver[36,37]. Conversely, a retrospective pooled cohort study of patients with CHB found that as the number of years of smoking cessation increased in ex-smokers, the rate of ALT elevation decreased. This effect is particularly potent after > 10 years of smoking cessation[38]. Previous research has provided an explanation on why smoking cessation reduces HCC. The ability of natural killer cells to stop HBV replication is known to be decreased by smoking, and notably, this effect is restored 1 mo after quitting smoking[39].
Some findings regarding the effect of exercise on HCC in patients with CHB are inconsistent, although the preventive effect of exercise on liver-related outcomes in patients with non-alcoholic fatty liver is well established. A study of patients with CHB revealed that exercise reduces the risk of HCC overall, whereas physical activity of ≥ 1500 MET-min/week increases the risk of HCC in patients with liver cirrhosis[13]. In addition, in a multinational cohort study, the effect of exercise on HCC prevention was not statistically significant in the sub-analysis of patients with CHB[40]. In our study, unlike alcohol abstinence and smoking cessation, regular exercise did not have a beneficial effect on HCC prevention or mortality. However, in this study, we had no detailed information on exercise, such as intensity or reason for exercise. Future studies are required to determine the effect of exercise and its type on HCC and the dose relationship in patients with CHB.
This study had several limitations. First, because this was a retrospective study, inevitable problems were encountered. Exclusion criteria were established to identify individuals who were expected to sustain their health status for an extended length of time at baseline. Nonetheless, the issue of reverse causation may remain. The K-NHIS does not provide information on the reasons for changing health behaviors. Consequently, sick quitter bias may arise in which people abstain from drinking or smoking because their health deteriorates to the point that they no longer find drinking or smoking enjoyable[41]. We further excluded participants who developed cancer or died within the first month of follow-up from baseline to minimize potential reverse causality. Second, our study used a self-report questionnaire to assess health behaviors. People might overreport or underreport health behaviors that would affect the results[42,43]. However, the NHSE uses a standardized questionnaire to assess health behaviors. Third, quantitative guidance for HCC risk and mortality reduction could not be offered because the dose- and time-effect linkages of LSM were not determined in this study. In addition, patients' assigned groups during the study might have changes, which could potentially underestimate the association between LSM and outcomes. However, additional biases in the analysis could have been introduced as some patients did not participate in the next health screening visit. Therefore, we performed target trial emulation to reduce selection bias and provide more robust estimates of treatment effects. Specifically, we were able to allocate the two groups at the “beginning” of induction, before subsequently emulating the randomization process using coarsened exact matching, through simultaneous determination of eligibility and assigning two arms. Fourth, we lacked several important variables for the outcome of CHB, for example, the degree of liver fibrosis and hepatitis B viral load. However, the administration of antiviral agents in Korea is subject to a reimbursement policy based on the presence of liver cirrhosis, elevated AST or ALT levels, and DNA levels. In this study, we attempted to partially account for liver fibrosis and DNA levels via adjusting for whether or not the patient received antiviral treatment. We were able to allocate the two groups at the “beginning” of induction, before subsequently emulating the randomization process using coarsened exact matching, through simultaneous determination of eligibility and assigning two arms.
In conclusion, LSM, particularly alcohol abstinence and smoking cessation, was associated with a reduced incidence of HCC in patients with CHB. Smoking cessation is also associated with reduced liver-related and all-cause mortality. Thus, active LSM should be encouraged in patients with CHB. A prospective study is required to confirm the results of this study and establish the dose and time effects of LSM in this population.
Despite various efforts to reduce the incidence of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB), it remains a persistent issue. Reports suggest that host factors such as alcohol consumption, smoking, and obesity, in addition to viral factors, contribute to the development of HCC.
Few studies have investigated the impact of lifestyle modifications (LSM) on reducing the incidence of HCC and liver-related mortality in patients with CHB.
This study used a nationwide database in Korea to investigate whether improvements in at least one of the following lifestyle factors - drinking, smoking, and sedentary behavior - were associated with reductions in HCC incidence and liver-related mortality in patients with CHB.
We analyzed patients with CHB who participated in the Korean National Health Insurance Service and who drank alcohol, smoked cigarettes, and were sedentary. Target trial emulation was used and covariates were adjusted through 2:1 propensity score matching.
With 48766 patients in the LSM group and 103560 in the control group, the LSM group had a lower adjusted hazard ratio for incident HCC and liver-related mortality compared to the control group. Within the LSM group, alcohol abstinence and smoking cessation were associated with lower adjusted hazard ratios for both outcomes.
LSM, specifically alcohol abstinence and smoking cessation, were associated with a decreased risk of HCC in patients with CHB.
The findings of this study suggest a need for active counseling for LSM in CHB patients, particularly alcohol abstinence and smoking cessation. Further investigation through a well-designed prospective cohort study is necessary to confirm these results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amin A, United Arab Emirates; Luo Y, China; Tai DI, Taiwan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 395] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 2. | Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471-2477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 4. | Jachs M, Tillmann HL. It's not all about the virus-On the importance of modifiable lifestyle factors in the development of hepatocellular carcinoma in chronic hepatitis B. Hepatology. 2023;77:352-354. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Wu S, Zhou J, Wu X, Sun Y, Wang B, Kong Y, Zhan S, Jia J, Yang HI, You H. Comparative Performance of 14 HCC Prediction Models in CHB: A Dynamic Validation at Serial On-Treatment Timepoints. Am J Gastroenterol. 2022;117:1444-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004;96:1851-1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Mak LY, Hui RW, Lee CH, Mao X, Cheung KS, Wong DK, Lui DT, Fung J, Yuen MF, Seto WK. Glycemic burden and the risk of adverse hepatic outcomes in patients with chronic hepatitis B with type 2 diabetes. Hepatology. 2023;77:606-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N, Kumada H. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 309] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Sinn DH, Kang D, Guallar E, Chang Y, Ryu S, Zhao D, Hong YS, Cho J, Gwak GY. Alcohol Intake and Mortality in Patients With Chronic Viral Hepatitis: A Nationwide Cohort Study. Am J Gastroenterol. 2021;116:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Chuang SC, Lee YC, Hashibe M, Dai M, Zheng T, Boffetta P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Kim JH, Sinn DH, Gwak GY, Kang W, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Insulin resistance and the risk of hepatocellular carcinoma in chronic hepatitis B patients. J Gastroenterol Hepatol. 2017;32:1100-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Fan R, Niu J, Ma H, Xie Q, Cheng J, Rao H, Dou X, Xie J, Zhao W, Peng J, Gao Z, Gao H, Chen X, Chen J, Li Q, Tang H, Zhang Z, Ren H, Cheng M, Liang X, Zhu C, Wei L, Jia J, Sun J, Hou J; Chronic Hepatitis B Study Consortium. Association of central obesity with hepatocellular carcinoma in patients with chronic hepatitis B receiving antiviral therapy. Aliment Pharmacol Ther. 2021;54:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chun HS, Park S, Lee M, Cho Y, Kim HS, Choe AR, Kim HY, Yoo K, Kim TH. Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Heckley GA, Jarl J, Asamoah BO, G-Gerdtham U. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11:446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F; Brescia HCC Study Group. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26:368-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 17. | Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates In Vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants (Basel). 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | El-Dakhly SM, Salama AAA, Hassanin SOM, Yassen NN, Hamza AA, Amin A. Aescin and diosmin each alone or in low dose- combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Res Notes. 2020;13:259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Abdu S, Juaid N, Amin A, Moulay M, Miled N. Effects of Sorafenib and Quercetin Alone or in Combination in Treating Hepatocellular Carcinoma: In Vitro and In Vivo Approaches. Molecules. 2022;27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 24] [Reference Citation Analysis (0)] |
| 20. | Nelson DR, Hrout AA, Alzahmi AS, Chaiboonchoe A, Amin A, Salehi-Ashtiani K. Molecular Mechanisms behind Safranal's Toxicity to HepG2 Cells from Dual Omics. Antioxidants (Basel). 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Al-Dabbagh B, Elhaty IA, Murali C, Madhoon AA, Amin A. Salvadora persicat; (Miswak): Antioxidant and Promising Antiangiogenic Insights. Am J Plant Sci. 2018;9:1228-1244. [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, Lee EJ, Ae Shin S. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 23. | Chun CB, Kim SY, Lee JY, Lee SY. Republic of Korea. Health system review. Health Systems in Transition 2009; 11. [Cited in This Article: ] |
| 24. | Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 651] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 25. | Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med. 2016;176:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of Screening Colonoscopy to Prevent Colorectal Cancer Among Medicare Beneficiaries Aged 70 to 79 Years: A Prospective Observational Study. Ann Intern Med. 2017;166:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Caniglia EC, Rojas-Saunero LP, Hilal S, Licher S, Logan R, Stricker B, Ikram MA, Swanson SA. Emulating a target trial of statin use and risk of dementia using cohort data. Neurology. 2020;95:e1322-e1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 399] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 29. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2771] [Cited by in F6Publishing: 3187] [Article Influence: 455.3] [Reference Citation Analysis (0)] |
| 30. | Luu HN, Behari J, Goh GB, Wang R, Jin A, Thomas CE, Clemente JC, Odegaard AO, Koh WP, Yuan JM. Composite Score of Healthy Lifestyle Factors and Risk of Hepatocellular Carcinoma: Findings from a Prospective Cohort Study. Cancer Epidemiol Biomarkers Prev. 2021;30:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2269] [Article Influence: 378.2] [Reference Citation Analysis (0)] |
| 32. | Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 655] [Cited by in F6Publishing: 598] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 33. | Purohit V, Rapaka R, Kwon OS, Song BJ. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci. 2013;92:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Oneta CM, Lieber CS, Li J, Rüttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010 . [PubMed] [Cited in This Article: ] |
| 36. | Tran TXM, Kim S, Song H, Park B. Longitudinal Changes in Smoking Habits in Women and Subsequent Risk of Cancer. Am J Prev Med. 2022;63:894-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 37. | Brahmania M, Liu S, Wahed AS, Yim C, Hansen BE, Khalili M, Terrault NA, Lok AS, Ghany M, Wang J, Wong D, Janssen HLA; Hepatitis B Research Network. Alcohol, tobacco and coffee consumption and liver disease severity among individuals with Chronic Hepatitis B infection in North America. Ann Hepatol. 2020;19:437-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Wang YH, Chuang YH, Wu CF, Jan MC, Wu WJ, Lin CL, Liu CJ, Yang YC, Chen PJ, Lin SM, Tsai MH, Huang YW, Yu MW. Smoking and Hepatitis B Virus-Related Hepatocellular Carcinoma Risk: The Mediating Roles of Viral Load and Alanine Aminotransferase. Hepatology. 2019;69:1412-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Meliska CJ, Stunkard ME, Gilbert DG, Jensen RA, Martinko JM. Immune function in cigarette smokers who quit smoking for 31 days. J Allergy Clin Immunol. 1995;95:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Baumeister SE, Schlesinger S, Aleksandrova K, Jochem C, Jenab M, Gunter MJ, Overvad K, Tjønneland A, Boutron-Ruault MC, Carbonnel F, Fournier A, Kühn T, Kaaks R, Pischon T, Boeing H, Trichopoulou A, Bamia C, La Vecchia C, Masala G, Panico S, Fasanelli F, Tumino R, Grioni S, Bueno de Mesquita B, Vermeulen R, May AM, Borch KB, Oyeyemi SO, Ardanaz E, Rodríguez-Barranco M, Dolores Chirlaque López M, Felez-Nobrega M, Sonestedt E, Ohlsson B, Hemmingsson O, Werner M, Perez-Cornago A, Ferrari P, Stepien M, Freisling H, Tsilidis KK, Ward H, Riboli E, Weiderpass E, Leitzmann MF. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J Hepatol. 2019;70:885-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Wannamethee G, Shaper AG. Men who do not drink: a report from the British Regional Heart Study. Int J Epidemiol. 1988;17:307-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 79] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Stockwell T, Zhao J, Greenfield T, Li J, Livingston M, Meng Y. Estimating under- and over-reporting of drinking in national surveys of alcohol consumption: identification of consistent biases across four English-speaking countries. Addiction. 2016;111:1203-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Kim Y, Choi YJ, Oh SW, Joh HK, Kwon H, Um YJ, Ahn SH, Kim HJ, Lee CM. Discrepancy between Self-Reported and Urine-Cotinine Verified Smoking Status among Korean Male Adults: Analysis of Health Check-Up Data from a Single Private Hospital. Korean J Fam Med. 2016;37:171-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |