Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3807
Peer-review started: February 2, 2023
First decision: March 20, 2023
Revised: March 30, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 28, 2023
Signet-ring cell carcinoma (SRCC) was previously thought to have a worse prognosis than other differentiated gastric cancer (GC), however, recent studies have shown that the prognosis of SRCC is related to pathological type. We hypothesize that patients with SRCC and with different SRCC pathological components have different probability of lymph node metastasis (LNM).
To establish models to predict LNM in early GC (EGC), including early gastric SRCC.
Clinical data from EGC patients who had undergone gastrectomy at the First Affiliated Hospital of Nanjing Medical University from January 2012 to March 2022 were reviewed. The patients were divided into three groups based on type: Pure SRCC, mixed SRCC, and non-signet ring cell carcinoma (NSRC). The risk factors were identified through statistical tests using SPSS 23.0, R, and Em-powerStats software.
A total of 1922 subjects with EGC were enrolled in this study, and included 249 SRCC patients and 1673 NSRC patients, while 278 of the patients (14.46%) presented with LNM. Multivariable analysis showed that gender, tumor size, depth of invasion, lymphovascular invasion, ulceration, and histological subtype were independent risk factors for LNM in EGC. Establishment and analysis using prediction models of EGC showed that the artificial neural network model was better than the logistic regression model in terms of sensitivity and accuracy (98.0% vs 58.1%, P = 0.034; 88.4% vs 86.8%, P < 0.001, respectively). Among the 249 SRCC patients, LNM was more common in mixed (35.06%) rather than in pure SRCC (8.42%, P < 0.001). The area under the ROC curve of the logistic regression model for LNM in SRCC was 0.760 (95%CI: 0.682-0.843), while the area under the operating characteristic curve of the internal validation set was 0.734 (95%CI: 0.643-0.826). The subgroups analysis of pure types showed that LNM was more common in patients with a tumor size > 2 cm (OR = 5.422, P = 0.038).
A validated prediction model was developed to recognize the risk of LNM in EGC and early gastric SRCC, which can aid in pre-surgical decision making of the best method of treatment for patients.
Core Tip: By establishing and comparing prediction models of lymph node metastasis (LNM) in early gastric cancer, we found that artificial neural network model was better than logistic regression model in sensitivity and accuracy. Among 249 signet-ring cell carcinoma (SRCC) patients, LNM was more common in mixed than in pure SRCC. A validated prediction model was also developed to recognize the risk for LNM in early gastric SRCC, which can be used to help make decisions regarding treatment of patients before performing surgery.
- Citation: Yang JJ, Wang XY, Ma R, Chen MH, Zhang GX, Li X. Prediction of lymph node metastasis in early gastric signet-ring cell carcinoma: A real-world retrospective cohort study. World J Gastroenterol 2023; 29(24): 3807-3824
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3807.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3807
According to the latest global cancer statistics, gastric cancer is considered to be the fifth leading type of cancer and the fourth leading cause of cancer-related deaths worldwide[1]. However, the progression of diagnostic methods has led to early gastric cancer (EGC) being more easily detectable[2]. Our previous study revealed that the rate of EGC diagnosis was 10.0%, while the rate of lymph node metastasis (LNM) in EGC was 12.3%[3]. EGC can be treated using surgical therapy with a 5-year survival rate of 80%-100%[4], and can also be resected via endoscopic resection, which is recommended as an effective method of treatment for EGC. Endoscopic submucosal dissection (ESD) can effectively improve the prognosis of patients[5]. Clinical decision of endoscopic vs surgical resection for EGC relies on the accurate assessment of the risk of LNM[6]. LNM mainly depends on tumor size, depth of invasion, and histological type[7].
Signet ring cell carcinoma (SRCC) is a common histological type with dismal prognosis[8] that predominantly consists of cells with poor cohesiveness with the predominance of cells in the signet ring. Among gastric cancer cases, the incidence of SRCC has increased and is reported to vary from 8.7% to 23.4%[9-11], while the behavior of SRCC in gastric cancer is controversial. In advanced gastric cancer, SRCC is considered to be a more severe invasive type with a higher level of peritoneal dissemination and a similar or worse prognosis than non-signet ring cell carcinoma (NSRC)[5,12,13]. However, it has been reported that the behavior of early SRCC may be equivalent to, or more favorable than other types of EGC due to its lower probability of LNM[14,15], suggesting that less invasive surgery is suitable. In addition, only few reports have been published on risk factors for LNM in early SRCC and the clinicopathological differences between pure and mixed SRCC.
Therefore, the objectives of this research study were to: (1) To identify the factors that can predict LNM in EGC to establish suitable models; and (2) to investigate the differences between pure and mixed SRCC to identify factors that can predict LNM in EGC with a signet-ring cell histology.
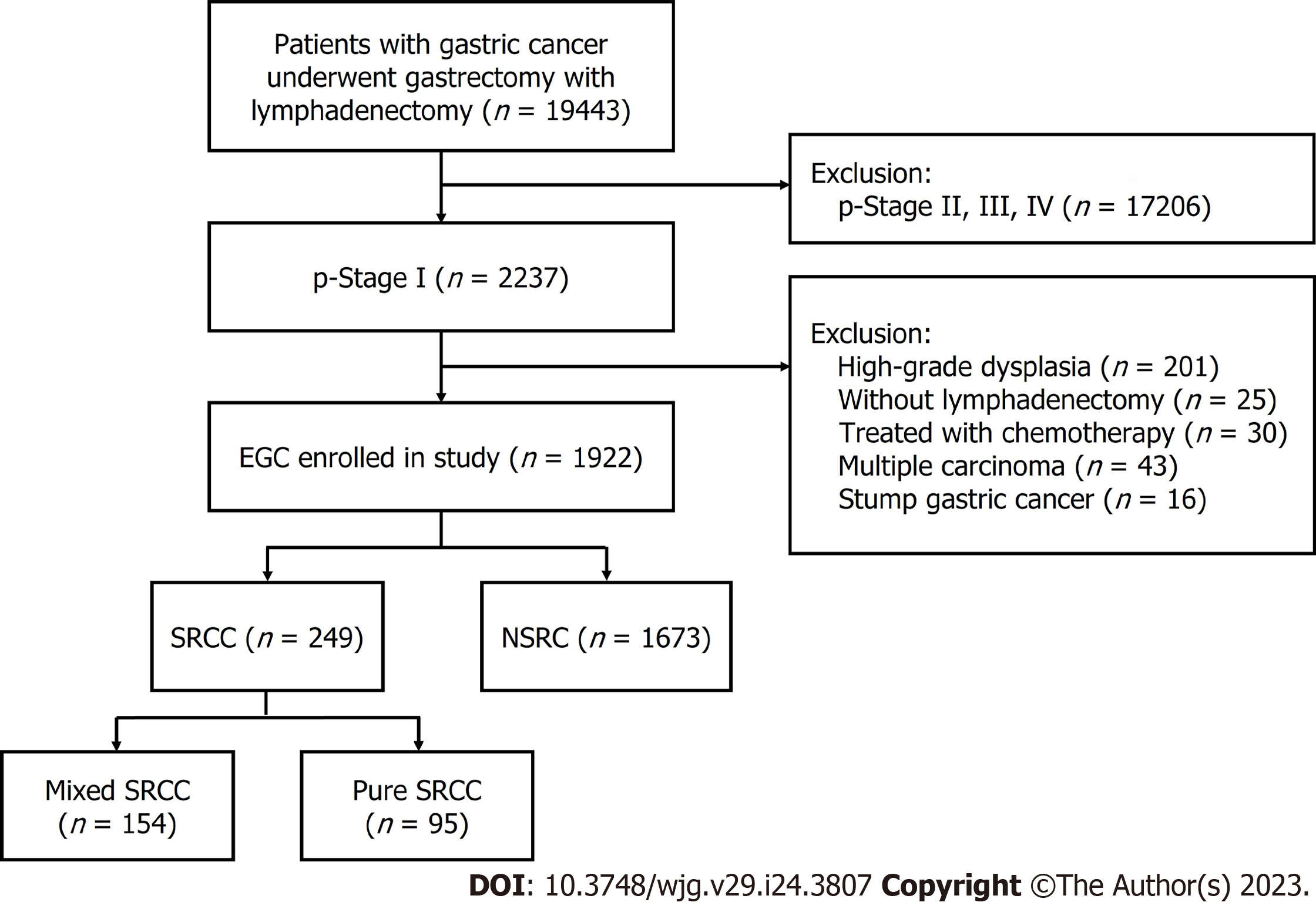
We examined the retrospective clinical information of patients who had undergone surgery at the First Affiliated Hospital of Nanjing Medical University, Nanjing, China, from January 2012 to March 2022. A total of 1922 patients were enrolled. The eligibility criteria used is illustrated in the flow diagram shown in Figure 1. Standard gastrectomy was the principal surgical procedure performed with a curative intent. Only patients who did not receive preoperative therapy were included in the study. The exclusion criteria were as follows: (1) pT2-4 gastric cancer identified using histopathological examination after radical gastrectomy; (2) high-grade dysplasia of the gastric mucosa; (3) a lack of lymphadenectomy; (4) cancer treated using neoadjuvant chemotherapy, (5) multiple gastric cancers; and (6) stump gastric cancer.
All resected tumors samples made into 2-mm-thick slices, stained with hematoxylin and eosin (H&E) and immunohistochemical staining, and assessed by qualified pathologists at the institution using World Health Organization (WHO) diagnostic criteria. Based on this, the patients were divided into 3 groups: (1) Pure SRCC (p-SRCC), which is defined as having a predominant component (> 50%) isolated carcinoma cells containing intracytoplasmic mucin16; (2) mixed SRCC (m-SRCC), which is defined as adenocarcinoma with a minor component (10-50%) of isolated carcinoma cells containing intracytoplasmic mucin; and (3) NSRC. Identification of risk factors associated with LNM in patients with EGC were conducted by comparing the clinicopathological characteristics of the patients and the gross findings of cancer lesions, including the age and gender, tumor size, location and macroscopic type, lymphovascular invasion (LVI), LNM, depth of invasion, perineural invasion, histological type, and ulceration during surgery. The patients were also divided into 5 macroscopic type groups: Protruded (I), elevate (IIa), flat (IIb), depressed (IIc), and excavated (III). As for the location of lesion, we divided the stomach into five sections: cardia, fundus, body, antrum, and angle.
LNM was determined based on the indications for ESD recommended by the Japanese Gastric Cancer Association. Based on the guidelines for ESD and endoscopic mucosal resection for EGC (2nd edition)[16], the absolute indications for endoscopic treatment are as follows: (1) Differentiated intramucosal carcinoma with a maximum diameter of ≤ 2 cm and without ulcerative lesions; (2) differentiated intramucosal carcinoma with a maximum diameter of > 2 cm and without ulcerative lesions; (3) cT1a with a diameter of ≤ 3 cm and ulceration [UL (+)]; and (4) undifferentiated intramucosal carcinoma with a maximum diameter of ≤ 2 cm and without ulcerative lesions. The terminology used in this study is based on the Japanese classification of gastric carcinoma.
Variables that are significantly associated with LNM (P < 0.05) were identified as candidate variables for the artificial neural network (ANN) model and multivariate logistic regression (LR). A random sample of 75% of all EGC patients were assigned to the training set, while the remaining 25% were used as internal validation of the LR model. The cut-off value was determined using ROC curves, which were then used to evaluate the discriminatory ability of the model. Then, a backward step-down selection process, with a maximum threshold of P < 0.05, was used for the final LR model selection of the nomogram. Bootstrapping using 2000 replications was conducted for internal validation.
In the ANN model, a total of 13 neural nodes were used as input layers. There were five neural nodes in the hidden layer, while the hyperbolic tangent transfer function was adopted. Two of the neural nodes in the output layer were LNM or not. The softmax transfer function was used as the transfer function. All samples were randomly assigned to the training group and the verification group at a ratio of 7:3. The training group was used to train the neural network. The verification group was used to evaluate the final neural network, and to construct the topological hierarchical structure of the neural network model. The final neural network fitting results were represented using the normalized importance percentage graph and a bar diagram was drawn. Finally, the area under the operating characteristic curve (AUC) of the recipients of the two models were calculated to compare and evaluate diagnostic accuracy using the chi-square test.
Continuous variables were converted to binary variables, and the cut-off point of age was determined by maximizing the sum of sensitivity and specificity after spline smoothing, which was found to be 65 years. Categorical variables are displayed as a percentage. Univariable analysis was performed to identify risk factors associated with LNM using various statistical tests, including the student t-test, Chi-square test, or the Fisher exact test. Multivariable analysis only included variables with a P < 0.05 in the univariate analysis. The results are shown as an odds ratio (OR), 95% confidence interval (CI), and a P value. The multivariate LR model was used to identify independent factors associated with LNM. Calibration of the model was assessed using the Hosmer-Lemeshow nomogram and ROC curve goodness-of-fit test. R (http://www.R-project.org) and EmpowerStats software (www.em
Among the 1922 patients enrolled, 249 patients were with SRCC and 1673 patients were with NSRC, and their outcomes are reported in Figure 1. The clinicopathological characteristics of SRCC and NSRC are described in Table 1. For patients with SRCC, the mean age was 53.14 ± 10.03, the male-to-female ratio was 1.15:1 (133/116), and the mean lesion size was 2.14 ± 1.43 cm. For patients with NSRC, the mean age was 57.01 ± 11.78, the male-to-female ratio was 2.85:1 (1238/435), and the mean lesion size was 1.89 ± 1.22 cm. When the patients were divided into five groups based on location, a majority of the EGCs were found to be in the gastric antrum and angle (n = 1189, 61.86%). There was a significant difference in SRCC and NSRC (P < 0.001) based on location. Most tumors were depressed (IIc), followed by flat (IIb) and elevated (IIa), protruded and excavated, which were in the minority, but no statistical significance was found based on macroscopic type between SRCC and NSRC (P = 0.105). Mucosa invasion occurred in 59.84% of (n = 149) SRCC, while submucosa invasion was observed more often in NSRC (n = 865, 51.70%). LVI was identified in 136 patients, among which 18 were diagnosed with SRCC and 118 were NSRC. LNM was identified in 278 patients, in which 62 were SRCC patients and 216 were NSRC patients. A total of 22 EGCs occurred with perineural invasion, of which 7 were SRCC and 15 were NSRC. Ulceration was identified in 701 patients, in which 74 (29.72%) were diagnosed with SRCC and 627 (37.48%) with NSRC. Overall, these results showed significant differences in age, gender, tumor size, LNM, depth of invasion, perineural invasion, tumor location, and ulceration between SRCC and NSRC.
| SRCC | NSRC | P value | |
| n = 249, n (%) | n = 1673, n (%) | ||
| Age (yr) | 53.14 ± 10.03 | 57.01 ± 11.78 | < 0.001 |
| ≤ 65 | 161 (64.66) | 634 (37.90) | |
| > 65 | 88 (35.34) | 1039 (62.10) | |
| Gender | < 0.001 | ||
| Male | 133 (53.41) | 1238 (74.00) | |
| Female | 116 (46.59) | 435 (26.00) | |
| Location | < 0.001 | ||
| Cardia | 11 (4.42) | 235 (14.05) | |
| Fundus | 5 (2.01) | 143 (8.55) | |
| Body | 43 (17.27) | 296 (17.69) | |
| Antrum | 129 (51.81) | 641 (38.31) | |
| Angle | 61 (24.50) | 358 (21.40) | |
| Macroscopic type | 0.105 | ||
| Protruded (0-I) | 3 (1.20) | 27 (1.61) | |
| Elevated (0-IIa) | 11 (4.42) | 128 (7.65) | |
| Flat (0-IIb) | 52 (20.88) | 353 (21.10) | |
| Depressed (0-IIc) | 178 (71.49) | 1076 (64.32) | |
| Excavated (0-III) | 5 (2.01) | 89 (5.32) | |
| Tumor size (cm) | 2.14 ± 1.43 | 1.89 ± 1.22 | 0.001 |
| ≤ 2 | 139 (55.82) | 1124 (67.18) | |
| > 2 | 110 (44.18) | 549 (32.82) | |
| Ulceration | 0.027 | ||
| UL (-) | 175 (70.28) | 1046 (62.52) | |
| UL (+) | 74 (29.72) | 627 (37.48) | |
| Depth of invasion | 0.001 | ||
| M | 149 (59.84) | 808 (48.30) | |
| SM | 100 (40.16) | 865 (51.70) | |
| LVI | 1 | ||
| Absence | 231 (92.77) | 1555 (92.95) | |
| Presence | 18 (7.23) | 118 (7.05) | |
| Perineural invasion | 0.041 | ||
| Absence | 242 (97.19) | 1658 (99.10) | |
| Presence | 7 (2.81) | 15 (0.90) | |
| LNM | < 0.001 | ||
| Absence | 187 (75.10) | 1457 (87.09) | |
| Presence | 62 (24.90) | 216 (12.91) |
In the univariate analysis, LNM was significantly associated with age (P = 0.015), gender (P < 0.001), tumor size (P < 0.001), macroscopic type (P = 0.008), depth of invasion (P < 0.001), LVI (P < 0.001), perineural invasion (P < 0.001), tumor location (P = 0.001), ulceration (P < 0.001) and histological subtype (P < 0.001). The multivariable analysis showed that being female (OR = 1.912, P < 0.001), tumor size (> 2 cm) (OR = 1.875, P = 0.001), submucosal invasion (OR = 3.340, P < 0.001), LVI (OR = 6.682, P < 0.001), the presence of ulcers (OR = 1.695, P = 0.001), and the pathological pattern of mixed SRCC (OR = 4.595, P < 0.001) were independent predictors of LNM in EGC (Table 2).
| LNM | Univariate | Multivariate analysis | ||||
| Present | Absent | P value | OR | 95%CI | P value | |
| Total | 278 | 1644 | ||||
| Age | 0.015 | |||||
| ≤ 65 | 135 | 660 | ||||
| > 65 | 143 | 984 | ||||
| Gender | < 0.001 | |||||
| Male | 160 | 1211 | 1 | |||
| Female | 118 | 433 | 1.912 | 1.392-2.626 | < 0.001 | |
| Location | 0.001 | |||||
| Cardia | 17 | 229 | ||||
| Fundus | 9 | 139 | ||||
| Body | 45 | 294 | ||||
| Antrum | 141 | 629 | ||||
| Angle | 66 | 353 | ||||
| Macroscopic type | 0.008 | |||||
| Protruded (0-I) | 9 | 21 | ||||
| Elevated (0-IIa) | 16 | 123 | ||||
| Flat (0-IIb) | 37 | 368 | ||||
| Depressed (0-IIc) | 201 | 1053 | ||||
| Excavated (0-III) | 15 | 79 | ||||
| Tumor size (cm) | < 0.001 | |||||
| ≤ 2 | 134 | 1129 | 1 | |||
| > 2 | 144 | 515 | 1.875 | 1.260-2.417 | 0.001 | |
| Ulceration | < 0.001 | |||||
| UL (-) | 134 | 1087 | 1 | |||
| UL (+) | 144 | 557 | 1.695 | 1.246-2.306 | 0.001 | |
| Depth of invasion | < 0.001 | |||||
| M | 54 | 903 | 1 | |||
| SM | 224 | 741 | 3.34 | 2.156-5.174 | < 0.001 | |
| LVI | < 0.001 | |||||
| Absence | 199 | 1587 | 1 | |||
| Presence | 79 | 57 | 6.682 | 4.358-10.245 | < 0.001 | |
| Perineural invasion | < 0.001 | |||||
| Absence | 262 | 1638 | ||||
| Presence | 16 | 6 | ||||
| Histological type | ||||||
| Pure SRCC | 8 | 87 | < 0.001 | 1 | < 0.001 | |
| NSRC | 216 | 1457 | 2.619 | 1.454-3.598 | 0.894 | |
| Mixed SRCC | 54 | 100 | 4.595 | 2.654-6.954 | < 0.001 | |
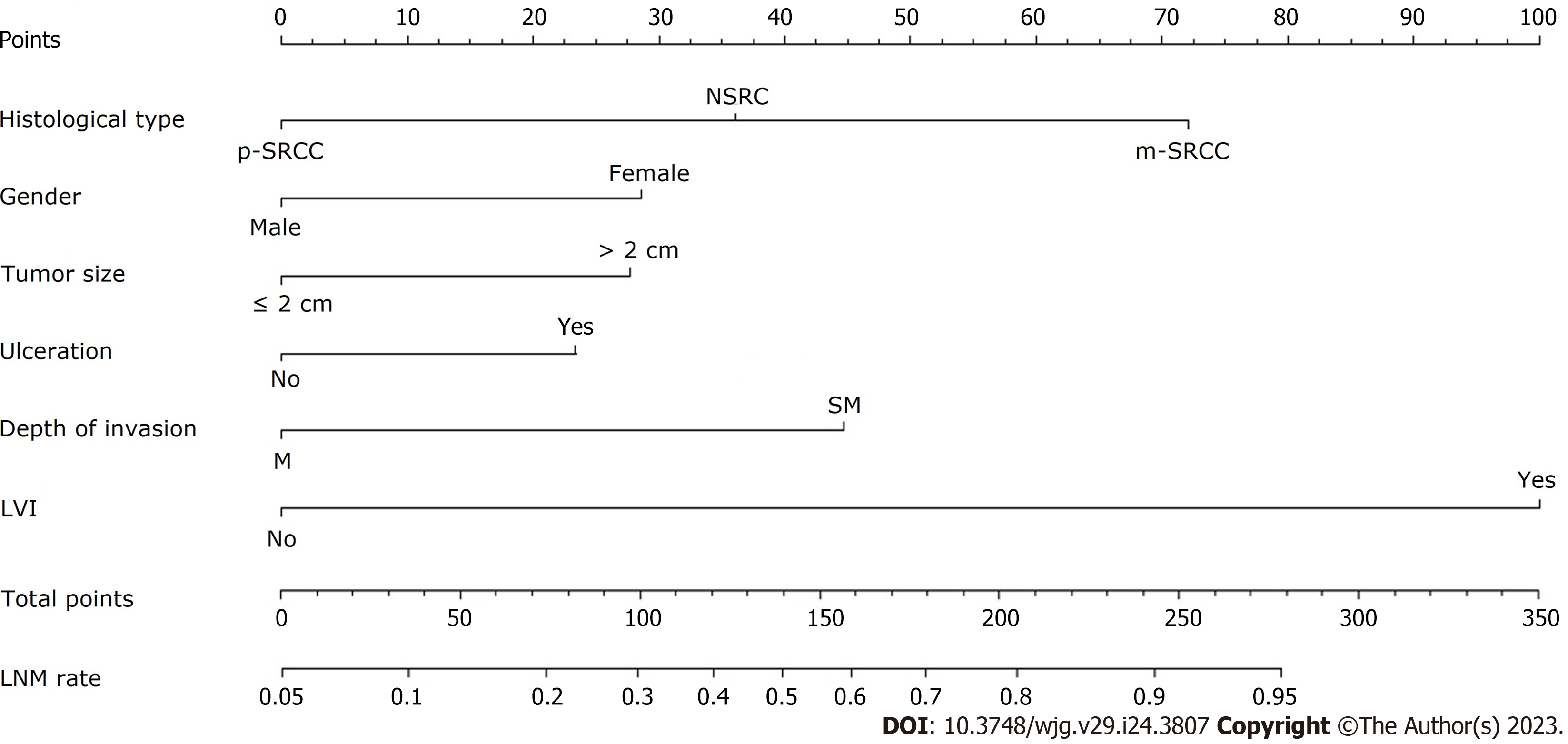
The LR model nomogram revealed that LVI had the greatest impact on scoring, followed by histological subtype and depth of invasion. The effects of gender, tumor size, and ulceration on model performance were also significant. Each level of the variable was summed using the total score based on the point scale and was positioned on the total score scale to determine the corresponding LNM probability for each patient (Figure 2). This predictive model demonstrated that the AUC was 0.797 with 95%CI: 0.776-0.818. Following 2000 bootstrap repetitions, the AUC of the internal validation in the training set was 0.816 (95%CI: 0.794-0.839).
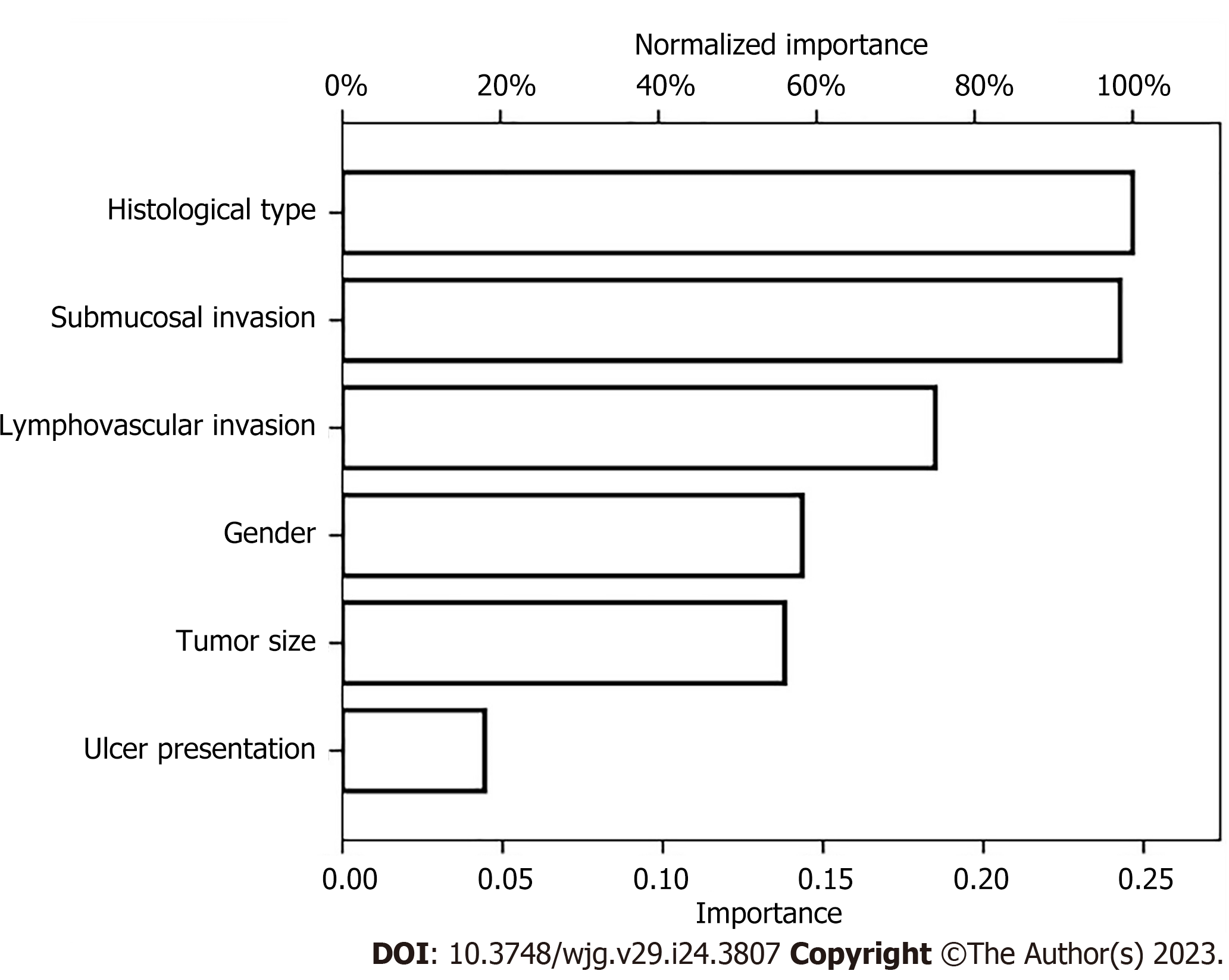
We also established an ANN model and assessed its ability by comparing it with a traditional LR model to predict the risk of LNM in EGC patients (Figure 3) presents the structure of the established ANN model and was influenced by 6 significant predictors: Gender, tumor size, submucosal invasion, LVI, histological type and ulceration, and histological type revealed the greatest impact on scoring, followed by the depth of invasion and LVI, while ulceration accounted for the least but still exerted a significant effect. The predictive model demonstrated an AUC of 0.878 with a 95%CI of 0.862-0.893.
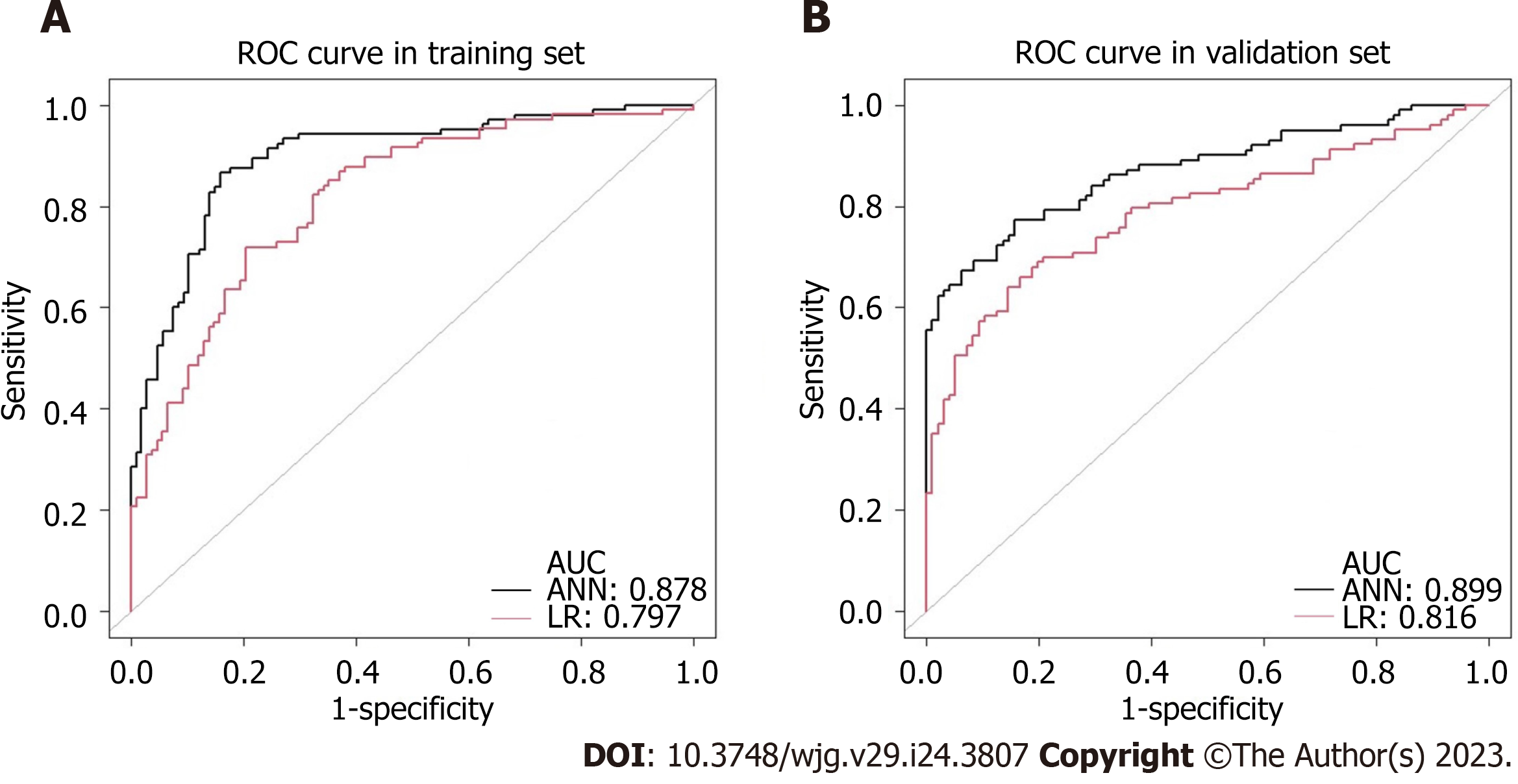
In addition, differences between ANN and the multivariate LR model were further compared based on sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and AUC according to the actual rate of LNM confirmed pathologically. Table 3 shows the classification distribution of the two models. The sensitivity and accuracy of the ANN model (96.4% vs 58.3%, P = 0.034, 88.2% vs 86.7%, P < 0.001) and the AUC (0.878 vs 0.797, P < 0.001; 0.899 vs 0.816, P < 0.001; respectively) were better than that of the multivariate LR model in the training and validation sets (Figure 4). The specificity (88.0% vs 88.8%, P < 0.001) and positive predictive value (19.4% vs 27.7%, P < 0.001) was inferior to that of the multivariate LR model. There was no difference in the negative predictive value between the two groups (99.9% vs 96.7%, P = 0.202). The comparisons of the diagnostic assessment are listed in Table 4.
| Models | Predicted results | Pathological diagnosis | ||
| + | - | Percent correct | ||
| LR | + | 77 | 55 | 58.3% |
| - | 201 | 1589 | 88.8% | |
| Percent correct | 27.7% | 96.7% | 86.7% | |
| ANN | + | 54 | 2 | 96.4% |
| - | 224 | 1642 | 88.0% | |
| Percent correct | 19.4% | 99.9% | 88.2% | |
| Diagnostic Index | LR model, (%, 95%CI) | ANN model (%, 95%CI) | P value |
| Sensitivity | 58.3% (48.6-67.1%) | 96.4% (87.8-99.9%) | 0.034 |
| Specificity | 88.8% (87.2-90.4%) | 88.0% (86.4-89.6%) | < 0.001 |
| PPV | 27.7% (22.3-33.9%) | 19.4% (14.9-25.2%) | < 0.001 |
| NPV | 96.7% (95.6-97.5%) | 99.9% (99.6-1.000%) | 0.202 |
| Accuracy | 86.7% (85.1-88.2%) | 88.2% (86.8-89.8%) | < 0.001 |
| AUC | 0.797 (0.776-0.818) | 0.878 (0.862-0.893) | < 0.001 |
As shown in Table 5, differences in the clinicopathological characteristics between pure SRCC (p-SRCC) and mixed SRCC (m-SRCC) patients were significant based on the following factors: (1) Age was younger in the p-SRCC (73.68%) patients than in the m-SRCC (59.09%) group (P = 0.037); (2) m-SRCC patients were more likely to show invasion into the SM layer, compared with p-SRCC (49.35% vs 25.26%, P < 0.001); (3) LVI resulted in a significantly higher proportion of cases in the m-SRCC (11.69% vs 0.00%, P = 0.002) group; (4) LNM was much more frequent in the m-SRCC group (35.06% vs 8.42%, P < 0.001) than in the p-SRCC group. We also found that ulcers were more likely to be present in the m-SRCC group than in the p-SRCC group, although the difference was no statistically significant. There were no significant differences in the other characteristics between the p-SRCC and m-SRCC groups.
| Variable | Pure SRCC | Mixed SRCC | P value |
| n = 95, n (%) | n = 154, n (%) | ||
| Age (yr) | 0.037 | ||
| ≤ 65 | 70 (73.68) | 91 (59.09) | |
| > 65 | 25 (26.32) | 63 (40.91) | |
| Gender | 0.131 | ||
| Male | 46 (48.42) | 87 (56.49) | |
| Female | 49 (51.58) | 67 (43.51) | |
| Location | 0.255 | ||
| Cardia | 4 (4.21) | 7 (4.55) | |
| Fundus | 2 (2.11) | 3 (1.95) | |
| Body | 13 (13.68) | 30 (19.48) | |
| Antrum | 51 (53.68) | 78 (50.65) | |
| Angle | 25 (26.32) | 36 (23.38) | |
| Macroscopic type | 0.152 | ||
| Protruded (0-I) | 1 (1.05) | 2 (1.30) | |
| Elevated (0-IIa) | 5 (5.26) | 6 (3.90) | |
| Flat (0-IIb) | 23 (24.21) | 29 (18.83) | |
| Depressed (0-IIc) | 65 (68.42) | 113 (73.38) | |
| Excavated (0-III) | 1 (1.05) | 4 (2.60) | |
| Tumor size (cm) | 0.126 | ||
| ≤ 2 | 60 (63.16) | 79 (51.30) | |
| > 2 | 35 (36.84) | 75 (48.70) | |
| Ulceration | 0.051 | ||
| UL (-) | 75 (78.95) | 100 (64.94) | |
| UL (+) | 20 (21.05) | 54 (35.06) | |
| Depth of invasion | < 0.001 | ||
| M | 71 (74.74) | 78 (50.65) | |
| SM | 24 (25.26) | 76 (49.35) | |
| LVI | 0.002 | ||
| Absence | 95 (100.00) | 136 (88.31) | |
| Presence | 0 (0.00) | 18 (11.69) | |
| Perineural invasion | 0.420 | ||
| Absence | 94 (98.95) | 148 (96.10) | |
| Presence | 1 (1.05) | 6 (3.90) | |
| LNM | < 0.001 | ||
| Absence | 87 (91.58) | 100 (64.94) | |
| Presence | 8 (8.42) | 54 (35.06) |
The univariate analyses demonstrated that the depth of invasion (M and SM), histological type and LVI differed significantly between patients with and without LNM in early SRCC patients. More frequent ulceration was observed in LNM positive SRCC patients, although the difference was not statistically significant. Based on the stepwise multivariate analysis, the significant independent risk factors for LNM in early SRCC were SM invasion (OR = 2.615, P = 0.008), LVI (OR = 14.903, P = 0.001), and a pathological pattern of mixed SRCC (OR = 1.982, P = 0.043). The independent risk factors are listed in Table 6. In addition, we also performed a risk analysis for LNM in SRCC limited to the mucosa and in SRCC with different histological types (p-SRCC and m-SRCC). In SRCC limited to the mucosa (Table 7), histological type (P = 0.010) and LVI (P = 0.016) were significantly different between patients with and without LNM, and the multivariate analysis showed that the pathological pattern of mixed SRCC (OR = 4.557, P = 0.023) was an independent predictor of LNM. In m-SRCC (Table 8), the univariate analyses showed that depth of invasion (M and SM) (P = 0.004) and LVI (P < 0.001) were risk factors for LNM, and that LVI (OR = 16.173, P = 0.001) was a significant independent risk factor for LNM. In p-SRCC, the analyses showed that patients with a tumor size that exceeded 2 cm (P = 0.014) and submucosal infiltration (P=0.045) were at a greater risk of LNM, indicating that tumor size > 2 cm (OR = 5.422, P = 0.038) is a significant independent risk factor for LNM (Table 8).
| LNM | Univariate | Multivariate analysis | ||||
| Present | Absent | P value | OR | 95%CI | P value | |
| Total | 62 | 187 | ||||
| Age | 0.617 | |||||
| ≤ 65 | 41 | 120 | ||||
| > 65 | 21 | 67 | ||||
| Gender | 0.639 | |||||
| Male | 32 | 101 | ||||
| Female | 30 | 86 | ||||
| Location | 0.544 | |||||
| Cardia | 2 | 9 | ||||
| Fundus | 1 | 4 | ||||
| Body | 10 | 33 | ||||
| Antrum | 33 | 96 | ||||
| Angle | 16 | 45 | ||||
| Macroscopic type | 0.209 | |||||
| Protruded (0-I) | 0 | 3 | ||||
| Elevated (0-IIa) | 1 | 10 | ||||
| Flat (0-IIb) | 13 | 39 | ||||
| Depressed (0-IIc) | 46 | 132 | ||||
| Excavated (0-III) | 2 | 3 | ||||
| Tumor size (cm) | 0.351 | |||||
| ≤ 2 | 30 | 109 | ||||
| > 2 | 32 | 78 | ||||
| Ulceration | 0.06 | |||||
| UL (-) | 37 | 128 | ||||
| UL (+) | 25 | 46 | ||||
| Depth of invasion | < 0.001 | |||||
| M | 20 | 129 | 1 | |||
| SM | 42 | 58 | 2.615 | 1.282-5.334 | 0.008 | |
| LVI | < 0.001 | |||||
| Absence | 46 | 185 | 1 | |||
| Presence | 16 | 2 | 14.903 | 3.143-70.680 | 0.001 | |
| Perineural invasion | 0.146 | |||||
| Absence | 58 | 184 | ||||
| Presence | 4 | 3 | ||||
| Histological type | < 0.001 | |||||
| Pure SRCC | 8 | 87 | 1 | |||
| Mixed SRCC | 54 | 100 | 1.982 | 0.923-4.237 | 0.043 | |
| LNM | Univariate | Multivariate analysis | ||||
| Present | Absent | P value | OR | 95%CI | P value | |
| Total | 20 | 129 | ||||
| Age | 0.195 | |||||
| ≤ 65 | 10 | 80 | ||||
| > 65 | 10 | 49 | ||||
| Gender | 0.306 | |||||
| Male | 9 | 77 | ||||
| Female | 11 | 52 | ||||
| Tumor size (cm) | 0.123 | |||||
| ≤ 2 | 8 | 78 | ||||
| > 2 | 12 | 51 | ||||
| Macroscopic type | 0.209 | |||||
| Protruded (0-I) | 0 | 2 | ||||
| Elevated (0-IIa) | 0 | 7 | ||||
| Flat (0-IIb) | 4 | 27 | ||||
| Depressed (0-IIc) | 15 | 91 | ||||
| Excavated (0-III) | 1 | 2 | ||||
| LVI | 0.016 | |||||
| Absence | 18 | 129 | ||||
| Presence | 2 | 0 | ||||
| Perineural invasion | - | |||||
| Absence | 20 | 129 | ||||
| Presence | 0 | 0 | ||||
| Location | 0.688 | |||||
| Cardia | 1 | 6 | ||||
| Fundus | 0 | 3 | ||||
| Body | 3 | 23 | ||||
| Antrum | 11 | 66 | ||||
| Angle | 5 | 31 | ||||
| Ulceration | 1 | |||||
| UL (-) | 15 | 101 | ||||
| UL (+) | 3 | 28 | ||||
| Histological type | 0.01 | |||||
| Pure SRCC | 3 | 66 | 1 | |||
| Mixed SRCC | 17 | 63 | 4.557 | 1.235-16.820 | 0.023 | |
| LNM | Univariate | Multivariate analysis | ||||||
| Present | Absent | P value | OR, 95%CI, P value | |||||
| Pure | Mixed | Pure | Mixed | Pure | Mixed | Pure | Mixed | |
| Total | 8 | 54 | 87 | 100 | ||||
| Age | 1 | 1 | ||||||
| ≤ 65 | 7 | 33 | 58 | 63 | ||||
| > 65 | 1 | 21 | 29 | 37 | ||||
| Gender | 1 | 0.377 | ||||||
| Male | 3 | 29 | 39 | 62 | ||||
| Female | 5 | 25 | 48 | 38 | ||||
| Location | 0.706 | 0.412 | ||||||
| Cardia | 0 | 2 | 4 | 5 | ||||
| Fundus | 0 | 1 | 2 | 2 | ||||
| Body | 1 | 9 | 12 | 21 | ||||
| Antrum | 5 | 28 | 46 | 50 | ||||
| Angle | 2 | 14 | 23 | 22 | ||||
| Macroscopic type | 0.788 | 0.314 | ||||||
| Protruded (0-I) | 0 | 0 | 1 | 2 | ||||
| Elevated (0-IIa) | 0 | 1 | 5 | 5 | ||||
| Flat (0-IIb) | 3 | 10 | 20 | 19 | ||||
| Depressed (0-IIc) | 4 | 42 | 61 | 71 | ||||
| Excavated (0-III) | 1 | 1 | 0 | 3 | ||||
| Tumor size (cm) | 0.014 | 0.751 | ||||||
| ≤ 2 | 4 | 26 | 56 | 53 | 1 | |||
| > 2 | 4 | 28 | 31 | 47 | 5.422 (1.095-26.856), 0.038 | |||
| Ulceration | 1 | 0.189 | ||||||
| UL (-) | 6 | 31 | 69 | 69 | ||||
| UL (+) | 2 | 23 | 18 | 31 | ||||
| Depth of invasion | 0.045 | 0.004 | ||||||
| M | 3 | 17 | 70 | 59 | 1 | |||
| SM | 5 | 37 | 17 | 41 | 2.395 (0.891-4.998), 0.054 | |||
| LVI | - | < 0.001 | ||||||
| Absence | 8 | 38 | 87 | 98 | 1 | |||
| Presence | 0 | 16 | 0 | 2 | 16.173 (4.085-76.619), 0.001 | |||
| Perineural invasion | 1 | 0.376 | ||||||
| Absence | 8 | 50 | 86 | 98 | ||||
| Presence | 0 | 4 | 1 | 2 | ||||
| Mixed component | 0.458 | |||||||
| Differentiated | - | 1 | - | 7 | ||||
| Undifferentiated | - | 30 | - | 58 | ||||
| Mixed | - | 23 | - | 35 | ||||
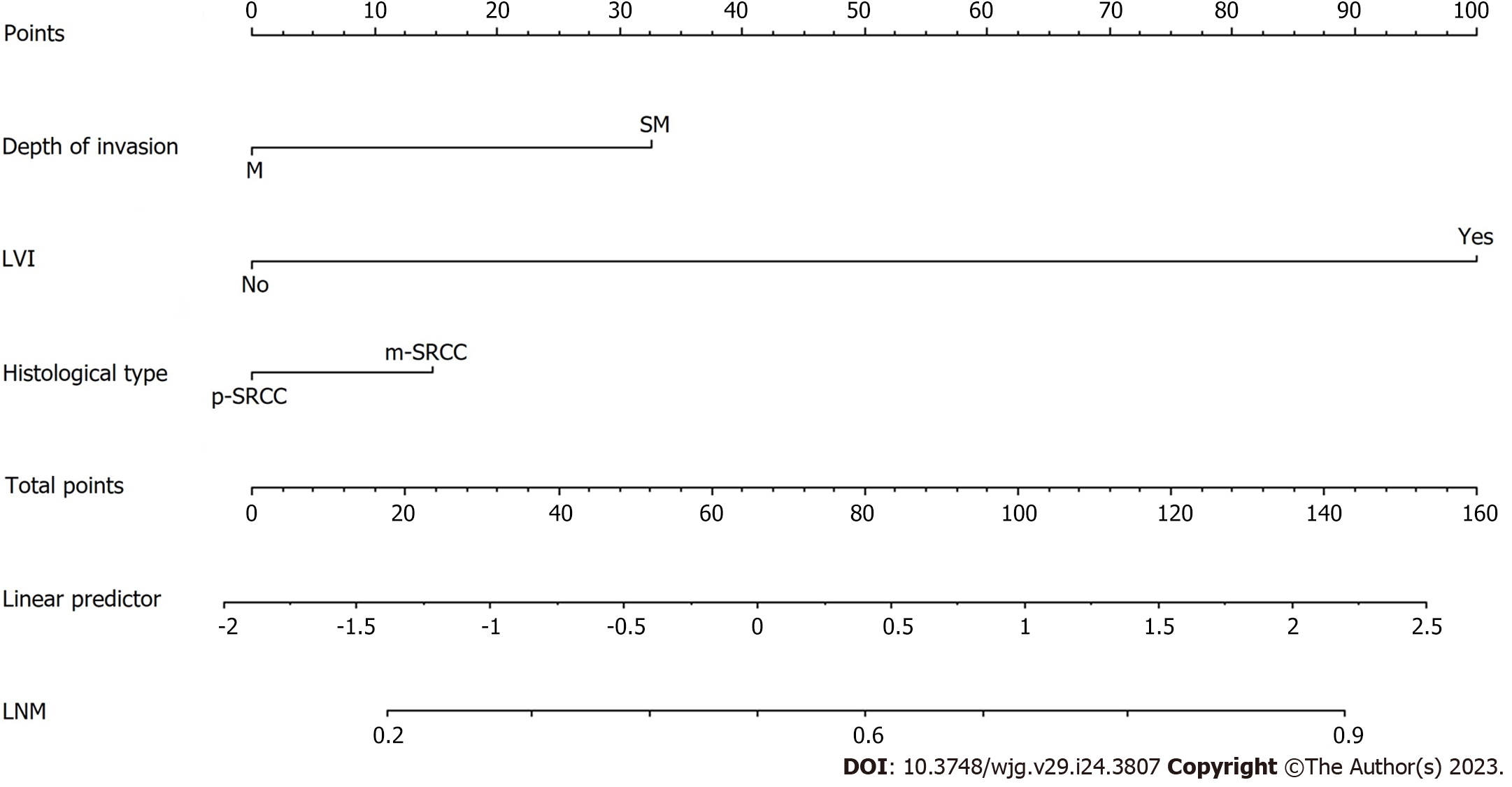
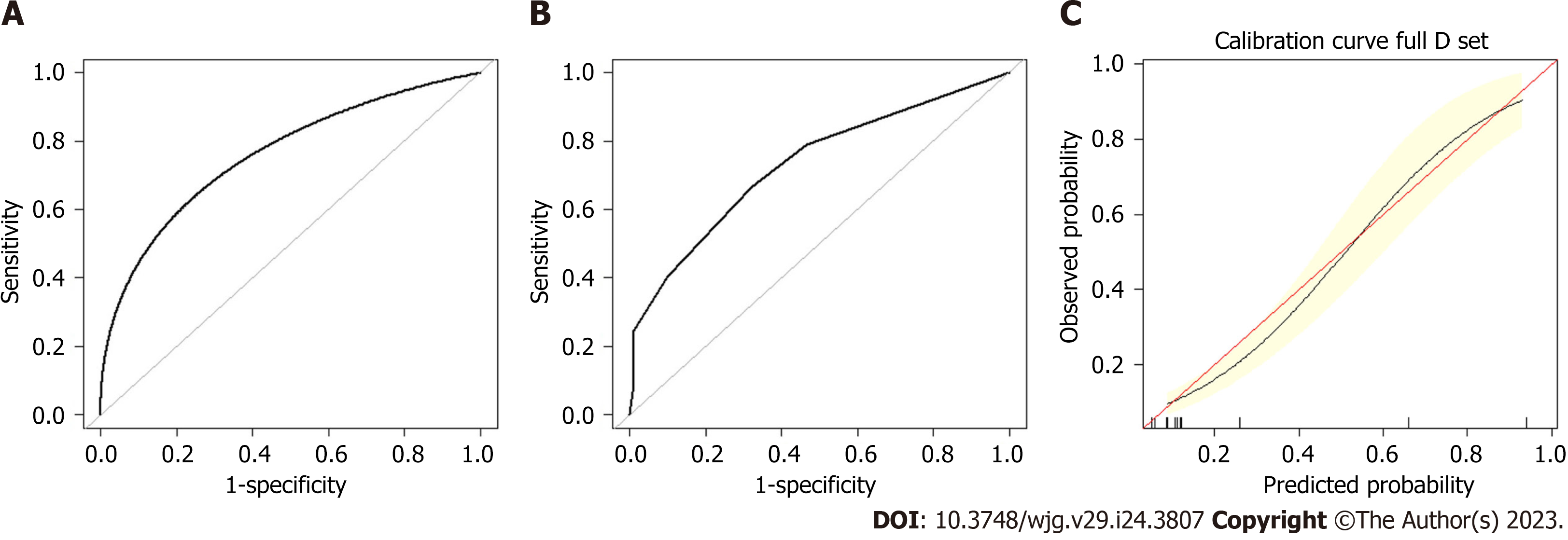
A preoperative predictive nomogram containing important risk factors related to early gastric SRCC LNM was constructed based on the LR model. All parameters of the clinicopathological risk factors of LNM in early gastric SRCC in the univariate analysis were included in the multivariable analysis. The nomogram revealed that LVI had the greatest impact on scoring, followed by invasion depth, and histological subtype (Figure 5). The equation stepwise selected prediction model from observed data included total risk points of LNM = -3.24513 + 0.93264*DEPTH + 2.86614*LVI + 0.42064*HISTOLOGICAL. This predictive model demonstrated an area under the curve (AUC) of 0.760 with a 95%CI of 0.682-0.843 (Figure 6A), while the AUC of internal validation was 0.734 (95%CI: 0.643-0.826) (Figure 6B). In the calibration plots, the bias-corrected line was calculated using 2000 bootstrapping iterations were indistinguishable and nearly approached the ideal line, while the 95%CI of the prediction model was also relatively accurate (Figure 6C). Therefore, the predictive power of the model was found to be good.
In the p-SRCC group, the LNM rate of patients with an expanded indication was 2.44% (1/41), and that of patients out of indication was 12.96% (7/54). In the m-SRCC group, the LNM rate in patients with an expanded indication was 16.13% (5/31), and in patients out of indication was 36.59% (45/123). The LNM rate between the expanded indication and out of indication patients in the m-SRCC group was statistically significantly different (P = 0.048), while there was no statistical difference in the p-SRCC group (P = 0.132). Among all 228 patients, the LNM rate between the expanded indication (6/72, 8.33%) and the out of indication (52/177, 29.38%) patients was statistically significantly different (P = 0.001) (Table 9).
| Meet expanded ESD indication | Beyond expanded ESD indication | P value | |||||
| Total | LNM (+) | LNM (-) | Total | LNM (+) | LNM (-) | ||
| p-SRCC | 41 | 1 | 40 | 54 | 7 | 47 | 0.132 |
| m-SRCC | 31 | 5 | 26 | 123 | 45 | 78 | 0.048 |
| Total | 72 | 6 | 66 | 177 | 52 | 125 | 0.001 |
As the fifth most common type of cancer worldwide, gastric cancer is a significant threat to human health. Recently, its incidence (mainly of the intestinal type) has declined in Asia, which may be associated with an increase in focus on and treatment using Helicobacter pylori in Asia. However, the incidence of SRCC is rising and needs more attention. The biological behavior of a case is very important when assessing whether ESD is feasible or not. However, the characteristics of early gastric SRCC, including LNM, clinicopathological features and prognosis, are still disputable[9,10,12]. Numerous reports have identified SRCC as an independent predictor of poor prognosis due to its specific characteristics, such as the high incidence of LNM, as well as the high rate of peritoneal carcinomatosis[17,18], and low sensitivity to chemotherapy[19], especially as the vast majority of these tumors are diagnosed at an advanced stage. Several studies have reported that tumors with a diameter > 2 cm, submucous infiltration, and lymphatic vascular infiltration are risk factors and independent risk factors for LNM in early gastric SRCC[19]. Huh et al[11] and Japanese Gastric Cancer Association[20] showed that the LNM rate was higher in early mixed SRCC than in early pure SRCC, and that mixed SRCC was more aggressive than pure SRCC. To reach an agreement on the treatment options for EGC and early gastric SRCC, these aspects need to be further explored.
A previous study found that being male, age, depressed type, submucosal invasion, LVI, and tumor location were independent risk factors for LNM in EGC[3]. Oh et al[21] demonstrated that in patients with EGC without LVI, a tumor size of > 3 cm, submucosal invasion, and undifferentiated histological type were significant risk factors for LNM. In our research study, the incidence of LNM was 14.46%, which is similar to that of previous studies[9,10,13,22-24], and we found that patients of EGC in younger (≤ 60), female, tumor size >2cm, with elevated macroscopic type, submucosal invasion, LVI, perineural invasion, tumor located in the gastric antrum and angle, ulceration, and a pathological pattern of mixed SRCC are more likely to have LNM, and all factors were statistically different. In the multivariate analysis, being female, tumor size of > 2 cm, with submucosal invasion, LVI, ulceration, and mixed SRCC were independent predictors of LNM in EGC.
Since SRCC shows different clinicopathological and biological characteristics, compared to NSRC, there are only a few studies that have previously reported on it. Therefore, we analyzed the clinicopathological and biological characteristics of SRCC, including pure-SRCC, mixed-SRCC, and SRCC limited to the mucosa in our research. By comparing the clinicopathological features of patients with NSRC and SRCC, we observed that patients with SRCC were of a younger age, were usually female, and showed fewer lesions in the upper section of the stomach with remarkable statistics differences. With respect to the risk factors for LNM in SRCC, our findings demonstrated that patients with a mixed type, submucosal invasion, and higher LVI are more likely to experience LNM. At the same time, we found that the mixed type was the only independent risk factor for LNM in patients with SRCC limited to the mucosa, which is consistent with the multivariable analysis of LNM in patients with mixed SRCC. In addition, the LNM rate in m-SRCC patients with an expanded ESD indication had a higher LNM rate than p-SRCC patients, and the difference was statistically significant. Therefore, for early gastric m-SRCC patients, caution should be exercised when selecting ESD treatment when the lesion exceeds the mucosal layer. For early gastric m-SRCC patients, a tumor size of > 2 cm was independent risk factor for LNM.
At present the predictive probability of LNM has not been clearly defined, and the clinical diagnosis of gastric LNM in EGC is still challenging. Quantitative predictive models are beneficial for both clinicians and patients in making more objective decisions regarding treatment options. The optimal threshold depends on the extent to which the patient or clinician rejects the risk. It is widely known that LR is a simple machine learning algorithm used for binary classification tasks. Neural networks are similar to a LR and can be referred to as a generalized LR. The neural network has advantages over LR models: the hidden layers facilitate the discovery of more complex and non-linear associations of variables. In our research study, we established two models: the ANN and LR model (nomogram) of LNM in EGC, and the LR model (nomogram) of LNM in early gastric SRCC patients. The ANN model and LR model both showed satisfactory performance through internal cross-validation. The methods of assessing diagnostic test accuracy were further used for model comparison. Then, we found that the ANN model of LNM in EGC performed better than the LR model with a significantly higher level of sensitivity, accuracy, and AUC. There were also some differences in the proportion of each risk factor in these two models of EGC. In the nomogram, LVI was dominant, and it was assigned 100 points; while in the ANN, the histological subtype was the most important risk factor, and it was assigned 100%. In addition, we established a nomogram of LNM in early gastric SRCC. In the nomogram, LVI was assigned 100 points; while, submucosal invasion was assigned 32.5 points, and mixed type SRCC was assigned 15 points. The potential of LNM gradually increased along with point accumulation. Our nomogram could predict the potential of LNM in every individual patient, which may help clinicians make informed and customized decisions that guide clinical treatment. Since only a few independent risk factors of LNM were obtained from the multivariate analysis, the ANN model cannot be further established in SRCC patients.
Our ANN and nomogram may serve as effective tools for predicting the incidence of LNM in Chinese patients with EGC, including early gastric SRCC, which may lead to improved selection of appropriate treatments methods. However, there are still several limitations in our study. Firstly, we utilized single-center, retrospective data to build and validate the predictive model. Secondly, we confirmed the predictability of the model only using internal validation. However, it is necessary to conduct external validation to demonstrate the accuracy of these models. Thirdly, only a limited number of participants were included in this study, which may lead to a statistical analysis bias. Finally, we did not develop a specific cut-off value for LNM for each of the different methods of treatment available for patients with EGC or early gastric SRCC. Hence, further multicenter, large-sample clinical studies that can help establish additional risk factors for LNM in EGC or early gastric SRCC are still necessary.
In conclusion, we constructed an ANN model and a nomogram to predict the probability of LNM in patients with EGC. The ANN model was better than the LR model nomogram in terms of sensitivity and accuracy. We constructed a nomogram for LNM in SRCC as well. These models can be used not only for preoperative evaluation to determine whether standard radical gastrectomy is needed for patients with EGC or early gastric SRCC patients at a high risk of LNM, but also for intraoperative evaluation to determine whether radical lymphadenectomy is necessary.
Signet-ring cell carcinoma (SRCC) has shown discriminative biological characteristics compared with adenocarcinoma, and the behavior of SRCC in gastric cancer is controversial. In this study, we recognize the risk of lymph node metastasis (LNM) in early gastric SRCC, which can aid in pre-surgical decision making of the best method of treatment for patients.
At present the predictive probability of LNM has not been clearly defined, and the clinical diagnosis of gastric LNM in early gastric cancer (EGC) and early gastric SRCC is still challenging. Quantitative predictive models are beneficial for both clinicians and patients in making more objective decisions regarding treatment options.
We aimed to establish models to predict LNM in EGC, including early gastric SRCC, which can aid in pre-surgical decision making of the best method of treatment for patients.
We examined the retrospective large-sample clinical information of patients who had undergone surgery, by comparing the clinicopathological features of patients with non-signet ring cell carcinoma and SRCC. Variables that are significantly associated with LNM were identified as candidate variables for the artificial neural network (ANN) model and multivariate logistic regression.
Our ANN and nomogram may serve as effective tools for predicting the incidence of LNM in Chinese patients with EGC, including early gastric SRCC, which may lead to improved selection of appropriate treatments methods.
With respect to the risk factors for LNM in SRCC, our findings demonstrated that patients with a mixed type, submucosal invasion, and higher LVI are more likely to experience LNM. At the same time, we found that the mixed type was the only independent risk factor for LNM in patients with SRCC limited to the mucosa, which is consistent with the multivariable analysis of LNM in patients with mixed SRCC.
Further multicenter, large-sample clinical and randomized controlled studies that can help establish additional risk factors for LNM in EGC or early gastric SRCC are still necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimofte GM, Romania; Muguruma N, Japan S-Editor: Yan JP L-Editor: A P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 45430] [Article Influence: 15143.3] [Reference Citation Analysis (47)] |
| 2. | Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Li X, Liu S, Yan J, Peng L, Chen M, Yang J, Zhang G. The Characteristics, Prognosis, and Risk Factors of Lymph Node Metastasis in Early Gastric Cancer. Gastroenterol Res Pract. 2018;2018:6945743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 6. | Hu Q, Dekusaah R, Cao S, Pang T, Wang Y, Zhang B, Lv Y, Zhang X, Ling T, Zhuge Y, Wang L, Zou X, Zhang W, Huang Q, Xu G. Risk Factors of Lymph Node Metastasis in Patients with Early Pure and Mixed Signet Ring Cell Gastric Carcinomas. J Cancer. 2019;10:1124-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 8. | Fléjou JF. [WHO Classification of digestive tumors: the fourth edition]. Ann Pathol. 2011;31:S27-S31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN, Hwang TL, Jan YY, Lin CJ. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Huh CW, Jung DH, Kim JH, Lee YC, Kim H, Yoon SO, Youn YH, Park H, Lee SI, Choi SH, Cheong JH, Noh SH. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 571] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, Yamauchi H. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Gronnier C, Messager M, Robb WB, Thiebot T, Louis D, Luc G, Piessen G, Mariette C; FREGAT working group-FRENCH. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 17. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Otsuji E, Yamaguchi T, Sawai K, Takahashi T. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 19. | Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C; FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684-93; discussion 693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 21. | Oh YJ, Kim DH, Han WH, Eom BW, Kim YI, Yoon HM, Lee JY, Kim CG, Kook MC, Choi IJ, Kim YW, Ryu KW. Risk factors for lymph node metastasis in early gastric cancer without lymphatic invasion after endoscopic submucosal dissection. Eur J Surg Oncol. 2021;47:3059-3063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, Min JS. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |