Published online Jun 14, 2023. doi: 10.3748/wjg.v29.i22.3422
Peer-review started: February 15, 2023
First decision: March 28, 2023
Revised: April 10, 2023
Accepted: May 11, 2023
Article in press: May 11, 2023
Published online: June 14, 2023
Hepatic fibrosis is a common pathological process of chronic liver diseases with various causes, which can progress to cirrhosis.
To evaluate the effect and mechanism of action annexin (Anx)A1 in liver fibrosis and how this could be targeted therapeutically.
CCl4 (20%) and active N-terminal peptide of AnxA1 (Ac2-26) and N-formylpep
Compared with the control group, AnxA1, transforming growth factor (TGF)-β1, interleukin (IL)-1β and IL-6 expression in the liver of mice with hepatic fibrosis induced by CCl4 was significantly increased, which promoted collagen deposition and expression of α-smooth muscle actin (α-SMA), collagen type I and connective tissue growth factor (CTGF), and increased progressively with time. CCl4 induced an increase in TGF-β1, IL-1β and IL-6 in liver tissue of AnxA1 knockout mice, and the degree of liver inflammation and fibrosis and expression of α-SMA, collagen I and CTGF were significantly increased compared with in wild-type mice. After treatment with Ac2-26, expression of liver inflammatory factors, degree of collagen deposition and expression of a-SMA, collagen I and CTGF were decrea
AnxA1 inhibited liver fibrosis in mice, and its mechanism may be related to inhibition of HSC Wnt/β-catenin pathway activation by targeting formylpeptide receptors to regulate macrophage function.
Core Tip: Hepatic stellate cells (HSCs) are the main receiver of inflammatory signals and the main promoter of fibrosis. Kupffer cells are activated by damage-associated molecular patterns or lipopolysaccharide, and other pathogen-associated molecular patterns, producing and releasing cytokines to activate HSCs, causing secretion and deposition of extracellular matrix and liver fibrosis. Annexin (Anx)A1 is a glucocorticoid regulatory protein that enhances efferocytosis in macrophages, inhibits activation of phospholipase A2, downregulates expression of inflammatory arachidonic acid, and negatively regulates inflammatory cells and factors. Whether AnxA1 is involved in the formation of liver fibrosis and its mechanism of action were the focus of this study.
- Citation: Fan JH, Luo N, Liu GF, Xu XF, Li SQ, Lv XP. Mechanism of annexin A1/N-formylpeptide receptor regulation of macrophage function to inhibit hepatic stellate cell activation through Wnt/β-catenin pathway. World J Gastroenterol 2023; 29(22): 3422-3439
- URL: https://www.wjgnet.com/1007-9327/full/v29/i22/3422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i22.3422
Various chronic liver diseases cause inflammation and necrosis of liver cells, and abnormal hyperplasia of fibrous connective tissue, leading to fibrosis, which is the early reversible stage of cirrhosis and one of the most important threats to human health worldwide[1,2]. When liver parenchyma is damaged, extracellular matrix (ECM) synthesis increases due to liver repair. When the cause is not removed, the continuously damaged liver leads to the failure of timely degradation of a large amount of synthetic ECM and excessive deposition in the liver tissue, which eventually develops into fibrosis[1-4]. The process involves a variety of fibrosis-promoting cells, including vascular smooth muscle cells, portal fibroblasts, and hepatic stellate cells (HSCs), and the activation and proliferation of HSCs is the main cell source of ECM[5-10]. Liver injury leads to necrosis or apoptosis of liver cells, and immune cells, mainly Kupffer cells are activated. Transforming growth factor (TGF)-β1, interleukin (IL)-1β, IL-6 and other cytokines are released, which promote the activation and proliferation of HSCs and secretion of inflammatory and fibrogenic factors. The activated HSCs express α-smooth muscle actin (α-SMA) and cytokines induce HSCs to differentiate into myofibroblasts, which secrete ECM components and cause liver fibrosis[5-11]. These changes may become an important target for the treatment of liver fibrosis.
Annexin (Anx)A1 is a member of the structurally related calcium-dependent phospholipid binding protein superfamily, which is widely distributed in all human tissues and cells, and involved in cell proliferation, differentiation, signal transduction, apoptosis, immunity and other physiological processes[12]. The proteins of the AnxA1 superfamily are homologous in structure, and each member has its unique N-terminal structure, which is related to its different functions and physiological effects[12]. After hydrolysis of full-length AnxA1, the N terminus is disconnected, and 26 amino acids in the N segment form a peptide chain fragment N-terminal peptide of AnxA1 (Ac2-26)[13]. AnxA1 and Ac2-26 both activate and bind to a specific G-protein-coupled receptor known as N-formylpeptide receptor (FPR)2[13], and Ac2-26 binds to FPR1[14]. AnxA1 inhibits expression of α-SMA and collagen I genes in TGF-β1-induced renal cortical fibroblasts[15] and regulates cigarette-smoke-induced pulmonary fibrosis[16]. However, whether AnxA1 regulates cell proliferation and collagen deposition in liver tissue remains unclear. The purpose of this study was to investigate the effects of AnxA1 on CCl4-induced inflammation, proliferation and collagen deposition in liver injury.
The Wnt/β-catenin signaling pathway is highly evolutionarily conserved and widespread in multicellular organisms. It is essential for cell growth, differentiation, ontogeny, genetic stability, apoptosis, self-renewal of stem cells, and homeostatic maintenance of adult tissues[17,18]. In normal liver tissue, the Wnt/β-catenin signaling pathway is almost inactive, but its abnormal activation plays an important role in the physiological and pathological processes of the liver. Increasing evidence has shown that abnormal expression of the Wnt/β-catenin signaling pathway is involved in the incidence of liver tumors, and the occurrence of liver inflammation, cirrhosis, and focal nodular hyperplasia[19]. AnxA1 has been reported as an important regulator of Wnt/β-catenin signaling[20], but whether AnxA1 contributes to the formation of liver fibrosis was the focus of this study.
Six-to-eight-week-old C57BL/6 mice (weighing 18-25 g; SLC, Guangxi, China) and C57BL/6JGpt Anxa1-KO mice (B6/JGptAnxa1em4Cd9634/Gpt, Strain NO. T016864; weighing 18-25 g; SLC, Jiangsu, China) were maintained in climate-controlled rooms under a 12-h light-dark cycle. All experiments were conducted in accordance with the institutional guidelines of Guangxi Medical University for the care and use of laboratory animals.
Thirty-two wild-type mice and 32 Anxa1-/- mice were each randomized into four subgroups of eight: Control, CCl4-induced liver fibrosis, liver fibrosis + Ac2-26, and liver fibrosis + Ac2-26 + FPR antagonist N-Boc-Phe-Leu-Phe-Leu-Phe (Boc-2). Mice in the control group received olive oil for intraperitoneal injection, while mice in the other three subgroups received 20% CCl4 (500 mL; Shanghai Acmec Biochemical Co., Ltd, China), and CCl4 + Ac2-26 1 mg/kg (500 mg; MedChemExpress, CAT: HY-P1098A/CS-0093919) with or without N-Boc2 1 mg/kg (500 mL; MedChemExpress, CAT: HY-103473A) for intraperitoneal injection twice weekly. Eight mice were used for each time point. At 4 and 8 wk after injection, animals were anesthetized with pentobarbital sodium (50 mg/kg intravenous injection), and blood samples were collected from the inferior vena cava. The livers were removed, and a small portion of liver tissue was fixed with 4% formaldehyde solution, and the remaining liver tissue was immediately frozen and stored in liquid nitrogen for RNA and protein extraction.
Hematoxylin-eosin (HE) staining and modified Masson three-color staining (Modified Masson’s Trichrome Staining Kit; Beijing Solarbio Science & Technology,) were performed after liver tissue fixation, dehydration, paraffin embedding, sectioning, dewaxing and hydration (bar: 50 mm).
The sections were dewaxed and hydrated according to AnxA1 monoclonal antibody instructions and SP-9000 Histostain™ - SP Kits (6 mL, Beijing Zhongshan Jinqiao Biotechnology, cat: K197712D/K196915F) requirements, antigenic alkaline repair, endogenous peroxidase blocking, goat serum blocking, and AnxA1 monoclonal antibody dilution 1: 1000 (Abcam, United Kingdom, cat: ab214486) were incubated overnight at 4 °C, the biotin-labeled goat anti-mouse/rabbit immunoglobulin G (IgG) (Invitrogen, United States, cat: TJ26211) was incubated for 15 min, and the horseradish-labeled Streptomyces vitellin was incubated for 15 min. Diaminobenzidine staining, counterstaining, dehydration, transparency, sealing, and microscopic examination. For each sample, five high-power fields (bar: 100 mm) were randomly selected, each containing an average of 400 cells, and the number of positive cells was counted for each field.
JS1 HSCs and AML1 hepatocytes were separately cultivated in Williams’ E medium containing 10% calf serum, 2 mmol/L L-glutamate and antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin). RAW246.7 cells were cultured in RPMI 1640 medium containing 10% calf serum and antibiotics. Cells were plated at (2 × 106)-(3 × 106)/mL and preincubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. The purity and viability of cells ranged from 87% to 95%. RAW246.7 cells HSCs and hepatocytes were incubated with 100 ng/mL lipopolysaccharide (LPS) (Sigma, St. Louis, MO, United States), LPS + 100 ng/mL Ac2-26, or LPS + Ac2-26 + 100 ng/mL Boc2 for 1, 3, 6, 12 and 24 h to activate expression of AnxA1, α-SMA, TGF-β1, connective tissue growth factor (CTGF), collagen I, IL-1β and IL-6. Ac2-26 or Ac2-26 + Boc2 dissolved in medium was added to the incubation medium 1 h prior to the addition of LPS.
Total RNA (1 μg) was extracted from liver or primary cells using TRIzol reagent (Takara Bio, Beijing, China) and reverse transcribed using a ReverTra Ace qPCR RT kit (Takara Bio). Target mRNA expression was quantified using quantitative real-time polymerase chain reaction (qPCR) as described previously[21]. TaqMan Gene Expression Assays for AnxA1 (forward primer 5’-AGCAGATCAAGGCCGCGTA-3’, reverse primer 5’-CATGGCACCACGGAGTTCA-3’), IL-6 (forward primer 5’-AGGATACCACTCCCAACAGACC-3’, reverse primer 5’-GCACAACTCTTTTCTCATTTCCAC-3’), TGF-β1 (forward primer 5’-TACGGCAGTGGCTGAACCAA-3’, reverse primer 5’-CGGTTCATGTCATGGATGGTG-3’), IL-1β (forward primer 5’-CACTACAGGCTCCGAGATGAACAAC-3’, reverse primer 5’-TGTCGTTGCTTGGTTCTCCTTGTAC-3’), α-SMA (forward primer 5’-CTGACAGAGGCACCACTGAA-3’, reverse primer 5’-CATCTCCAGAGTCCAGCACA-3’), collagen I (forward primer 5’-AAGAGGCGAGAGGTTTCC-3’, reverse primer 5’-AGAACCATCAGCACCTTTGG-3’), CTGF (forward primer 5’-CAAAGCAGCAAATACCA-3’, reverse primer 5’-GGCCAAATGTGTCTTCCAGT-3’), and GAPDH (forward primer 5’-TGTGTCCGTCGTGGATCTGA-3’, reverse primer 5’-TTGCTGTTGAAGTCG
Protein samples (30-50 μg) were prepared from livers and cells and separated by SDS-PAGE, followed by transfer to polyvinylidene difluoride membranes as reported previously[21]. Blots were incubated with antibodies (1:1000) against AnxA1 (Abcam, cat: ab214486), α-SMA (50 μL, Affinity Biosciences, Jiangsu, cat: AF1032), β-catenin (50 mL, MedChemExpress, cat: 51067-2-AP) and GAPDH (Abcam, cat: ab181602), followed by incubation with a peroxidase-conjugated goat anti-mouse/rabbit IgG(H + L) (1:5000; Invitrogen, cat: TJ26211). Proteins were visualized using fluorescence scanner imaging system (Odyssey; Bio-Rad, Hercules, CA, United States).
Results are expressed as mean ± SE (n = 8), unless otherwise indicated. Statistical analyses were performed using Kruskal-Wallis one-way analysis of variance. P < 0.05 was considered significant.
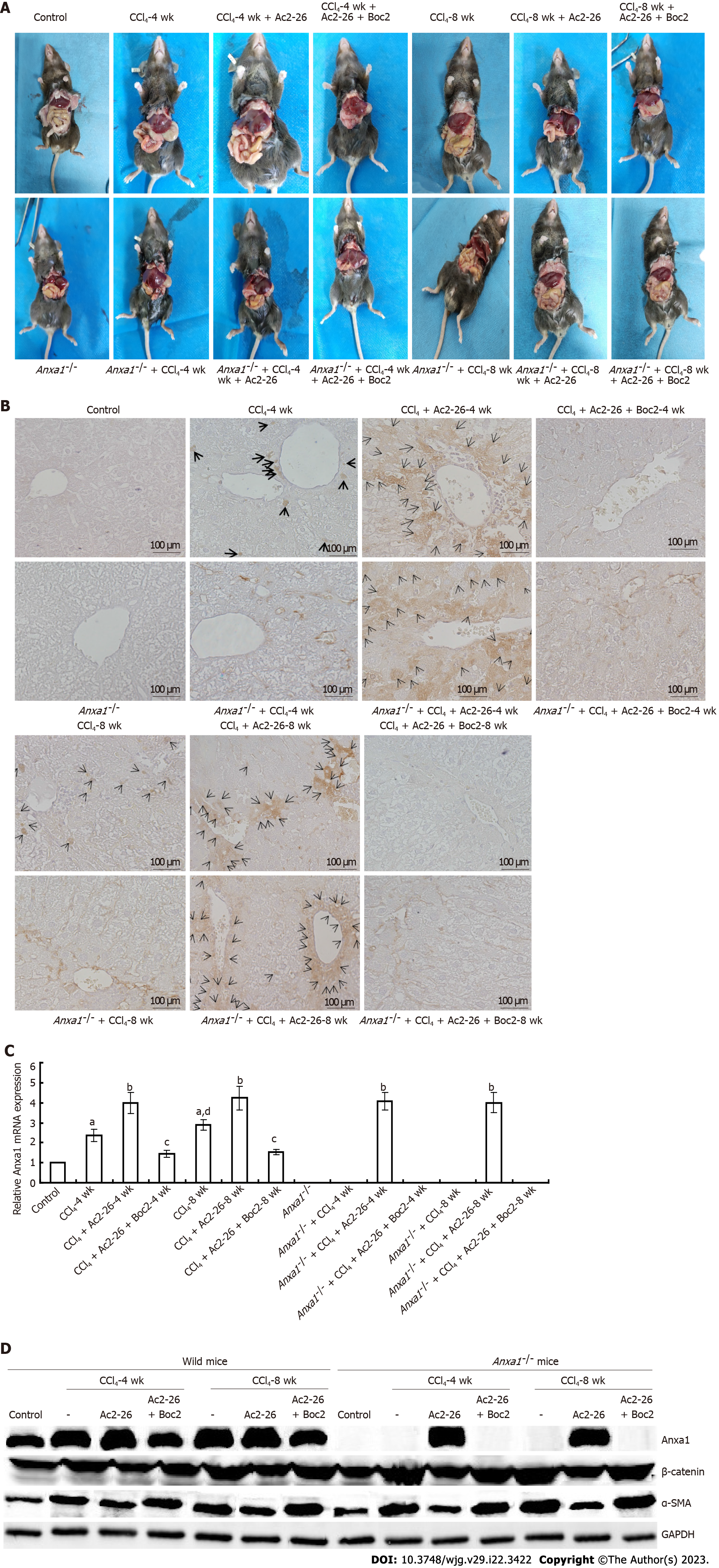
The liver of the control mice was red with bright color and smooth surface. In the CCl4-induced wild-type mice group, the liver tissue became light in color, rough on the surface, dull around the edge, and hard in texture, and nodules or even cellulose-like changes appeared over time. The lesions in the AnxA1-/- group were more severe than in the wild-type mice. After treatment with Ac2-26, the appearance of the liver in the CCl4-induced mice was significantly improved with dark red color, and the surface roughness and nodulation were significantly reduced, the effects of Ac2-26 were inhibited by Boc2 (Figure 1A). Compared with the control group, AnxA1 expression was significantly increased in the CCl4 model group, and was higher at 8 wk than 4 wk, suggesting that AnxA1 was related to the development of liver fibrosis. AnxA1 expression was higher in liver tissues of the CCl4 model group after Ac2-26 treatment, while Boc2 inhibited AnxA1 expression (Figures 1B and C), and the difference was significant (P < 0.05). Western blotting and qPCR results were consistent with immunohistochemical results (Figure 1D).
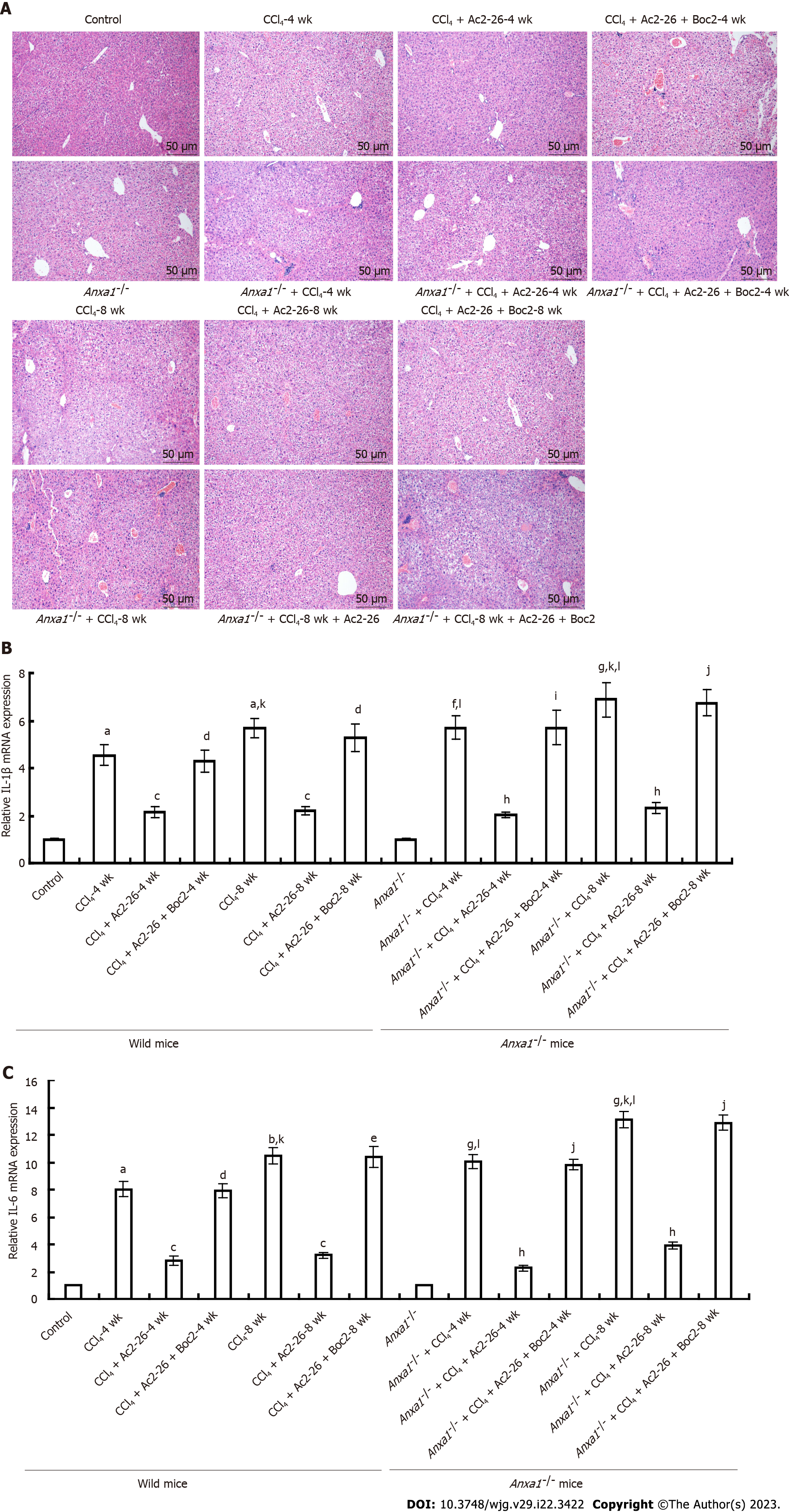
HE staining showed that the structure of hepatic lobules in the control group was clear, and there was no vacuole formation or obvious inflammatory cell infiltration. In the CCl4-induced wild model group, HE staining for 4 wk showed that the hepatocytes were swollen and enlarged, with scattered degeneration and necrosis, as well as infiltration of monocytes and lymphocyte cells. Degeneration and necrosis of hepatocytes were observed at 8 wk. In the AnxA1-/- model group with fibrosis induced by CCl4, liver tissue destruction was accompanied by a large number of inflammatory cell infiltration and steatosis. At 4 wk, there was massive necrosis of liver cells, massive infiltration of inflammatory cells, and disorder of hepatic lobular structure. At 8 wk, there was obvious structural destruction that became more severe over time. The infiltration of liver inflammatory cells and destruction of liver lobular structure in the AnxA1-/- mice were more obvious than those in the wild-type mice. After treatment with Ac2-26, the damage to the hepatic lobular structure and inflammatory cell infiltration in the CCl4-induced mice was reduced compared with that before treatment. AnxA1 mimics the peptide Ac2-26, which largely controls inflammation and is protective against hepatic fibrosis. In the model group treated with Ac2-26 and Boc2, Boc2 almost completely eliminated the effects of Ac2-26 at 4 wk (Figure 2A), suggesting that AnxA1 inhibited CCl4-induced liver tissue injury and reduced inflammatory cell infiltration. AnxA1 works by targeting mainly FPR receptors.
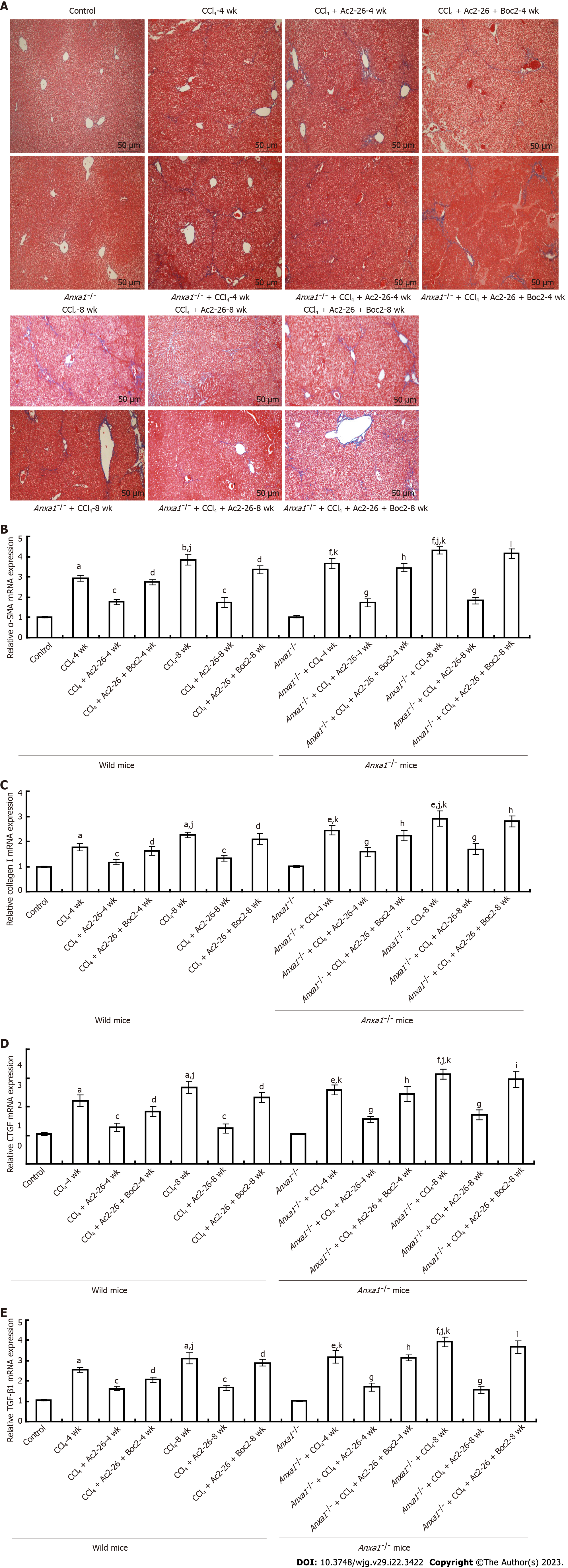
Collagen deposition is an important indicator of the degree of liver fibrosis, and can be visualized by Masson staining. Staining clearly showed that there was no collagen staining in the control group of wild-type and AnxA1-/- mice. In the CCl4-induced wild model group, blue collagen fibers proliferated in the portal area at 4 wk, but the degree of collagen fibrosis was mild. Similarly, the proliferation of blue-stained collagen fibers in the portal area was observed in the wild model group at 8 wk, but the collagen fibers were further increased, widened and extended to the surrounding hepatic lobular space than at 4 wk, suggesting that the liver fibers were gradually aggravated with the extension of modeling time. Compared with wild model mice, CCl4 induced more significant collagen deposition in the liver tissue of AnxA1-/- mice. At 4 wk, blue collagen fibers proliferated obviously in hepatic portal area and began to extend to liver parenchyma, and collagen fibers began to segment liver parenchyma. At 8 wk, a large amount of collagen surrounded the liver cells to form pseudolobules, which seriously damaged the normal structure of the liver tissue, indicating formation of fibrosis. The collagen deposition in the liver of AnxA1-/- mice was significantly aggravated by CCl4, and the degree of liver fibrosis was even more severe. The collagen deposition and wrapping in the liver of mice in the CCl4-induced mice was significantly weakened after Ac2-26 treatment, and the severity of liver fibrosis was relieved. In the model group, the damage to the liver tissue structure and collagen deposition after treatment with Ac2-26 and Boc2 were similar to those before treatment (Figure 3A).
α-SMA is a marker of HSC activation and transformation into fibroblasts. HSC activation generates and secretes ECM, and type I collagen is the ECM marker of HSCs. In this study, qPCR and western blotting were used to detect the expression levels of α-SMA and collagen I in liver tissue, and to evaluate the activation status of HSCs. Expression of α-SMA and type I collagen in the liver of wild-type mice was significantly increased by CCl4, while expression of α-SMA and collagen I in the liver of mice in the AnxA1-/- group was higher and increased with time, which was in accordance with the progression of liver fibrosis in the CCl4-induced mice. All of the above effects were inhibited by Ac2-26, suggesting that AnxA1 inhibited HSC activation and collagen deposition. In contrast, Boc2 reversed the therapeutic effect of Ac2-26 (Figures 3B and C), and the difference was significant (P < 0.05). Western blotting and qPCR results were consistent with immunohistochemical results (Figure 1D).
CCl4 induces expression of various cytokines in liver tissues, among which IL-1β, IL-6 and TGF-β1 are important factors leading to the formation of fibrosis. Expression of IL-1β, IL-6 and TGF-β1 in the liver of the CCl4-induced wild model group was increased simultaneously, and upregulation of inflammatory cytokines was more obvious and increased with time in the AnxA1-/- model group, while the Ac2-26 significantly inhibited expression of IL-1β, IL-6 and TGF-β1. Boc2 inhibited the anti-inflammatory effect of Ac2-26 (Figures 2B, 2C and 3E), and the difference was significant (P < 0.05). This suggested that AnxA1 exerted its anti-inflammatory and antifibrotic effects by inhibiting expression of TGF-β1, IL-1β and IL-6 through FPR.
CTGF mediates TGF-β1 promotion of ECM production and accumulation. CTGF regulates the growth of fibroblasts and epidermal cells, promoting cell adhesion, proliferation and ECM synthesis. Compared with the control group, the concentration of CTGF in the liver tissue of the CCl4-induced wild model group and AnxA1-/- model group was higher, and paralleled the progression of liver fibrosis. The more severe the fibrosis, the higher the expression of CTGF, suggesting that CTGF was closely related to the occurrence and development of liver fibrosis. After Ac2-26 treatment, expression of CTGF in liver tissues of mice in the CCl4-induced mice was significantly decreased. AnxA1 inhibited expression of CTGF in liver tissue and alleviated the occurrence of liver fibrosis, while Boc2 almost reversed the therapeutic effect of Ac2-26 (Figure 3D), and the difference was significant (P < 0.05).
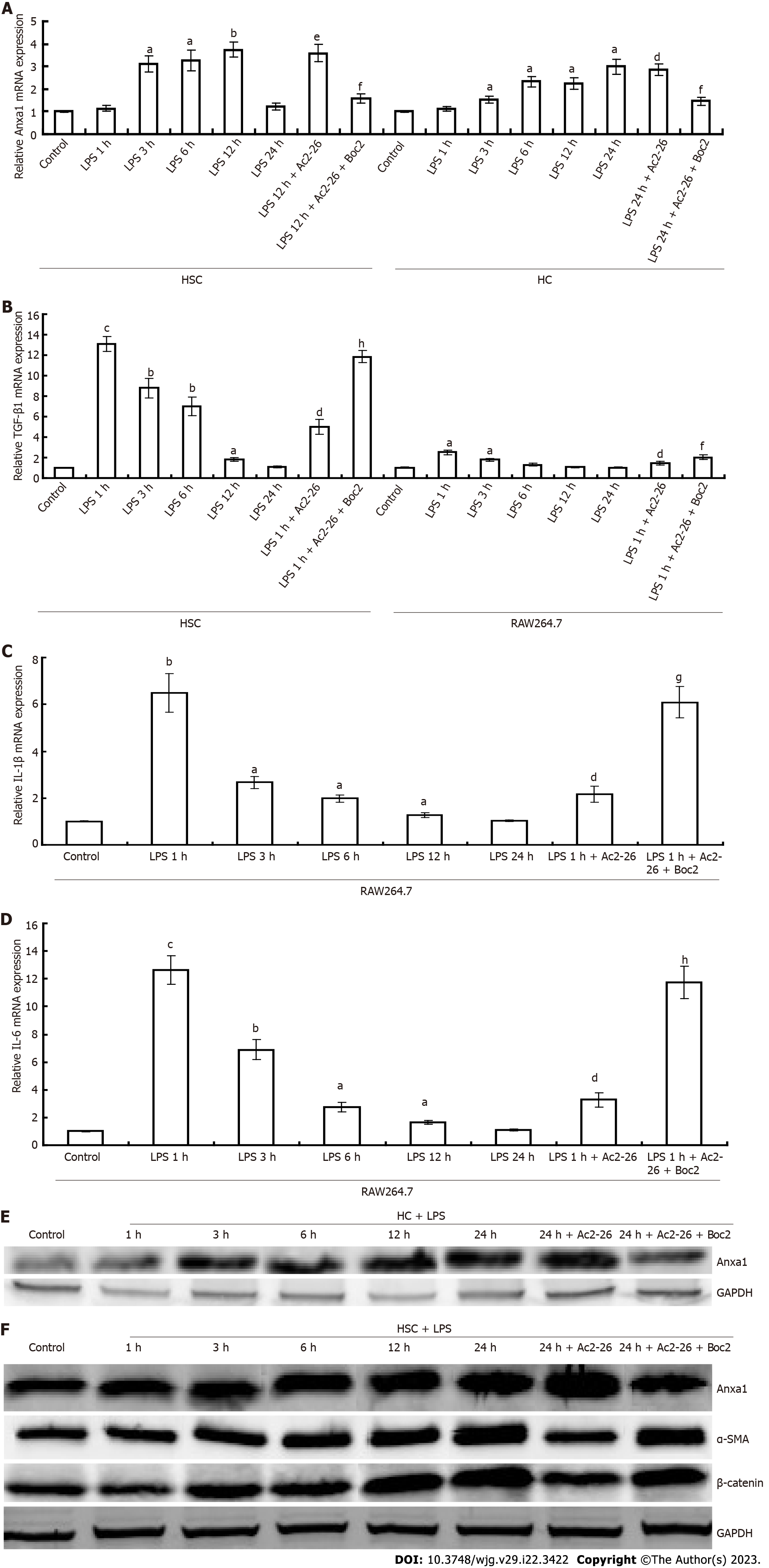
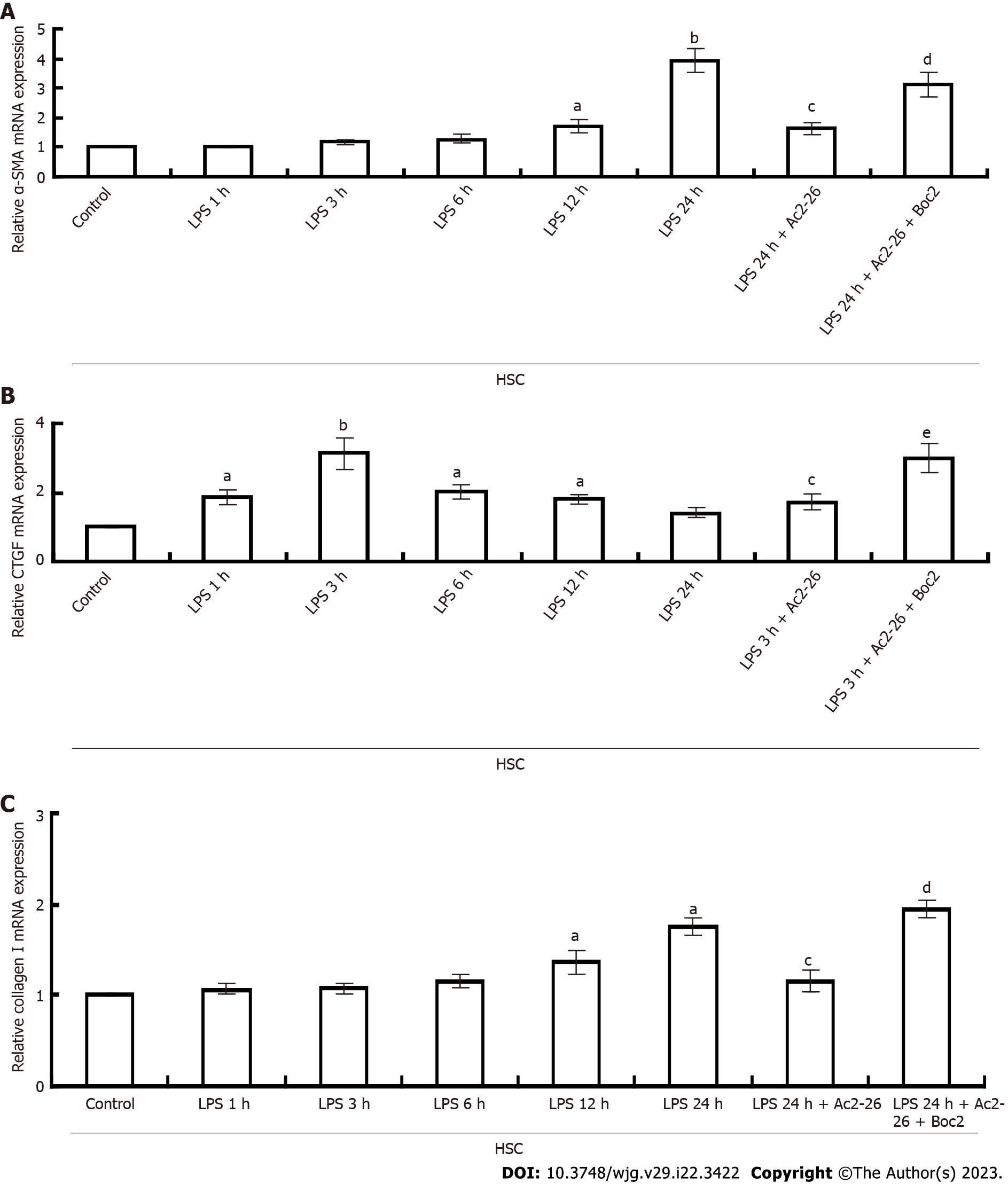
LPS induced activation of RAW264.7 cells and the release of inflammatory cytokines. Compared with the control group, expression of TGF-β1, IL-1β and IL-6 in RAW264.7 cells was significantly induced by LPS, and reached a peak within 1 h, then gradually decreased to the normal level. After activation of RAW264.7 cells, AnxA1 was not significantly increased, but Ac2-26 inhibited TGFβ1, and IL-6 expression in RAW264.7 cells, while Boc2 blocked the inhibitory effect of Ac2-26 on RAW264.7 cells (Figures 4B-D), and the difference was significant (P < 0.05). LPS induced activation of HSCs, which expressed TGFβ1, α-SMA, CTGF, and collagen I simultaneously (Figures 4B and 5A-C). Expression of TGF-β1 and CTGF began to increase at 1 h and peaked at 3 and 6 h, respectively. AnxA1 expression began to increase at 3 h and peaked at 12 h. Expression of α-SMA and collagen I began to increase significantly at 12 h and peaked at 24 h. Ac2-26 treatment significantly decreased LPS-induced expression of TGF-β1, α-SMA, CTGF and collagen I, and Boc2 significantly inhibited this effect (Figures 4B and 5A-C and the difference was significant (P < 0.05). LPS induced activation of hepatocytes. Compared with the control group, AnxA1 was highly expressed after hepatocyte (HC) activation, which was time-dependent and increased from 4 to 24 h (Figure 4A). Ac2-26 significantly upregulated HSC AnxA1 expression, while Boc2 inhibited AnxA1 expression (Figure 4A), and the difference was significant (P < 0.05). The results of western blotting and qPCR were consistent (Figures 4E and F).
The Wnt/β-catenin pathway plays an important role in the formation of liver fibrosis. We used western blotting to detect β-catenin expression, which was associated with inflammation and fibrosis. Compared with the low expression of β-catenin in control mice, expression of β-catenin was upregulated in the model group and higher in the AnxA1-/- group, and expression was higher at 8 wk than at 4 wk. After Ac2-26 treatment, expression of β-catenin protein in the CCl4-induced model group was significantly decreased compared with that before treatment, while Boc2 inhibited the effect of Ac2-26 (Figure 1D). The results of the animal experiments were verified by in vitro cell experiments. β-catenin was highly expressed in LPS-induced HSC activation, and increased progressively with time, starting at 3 h and reaching a peak at 24 h. Ac2-26 inhibited expression of β-catenin protein in LPS-induced HSCs, while Boc2 inhibition blocked this effect, β-catenin protein expression was maintained at a high level in LPS-induced HSCs (Figure 4F).
Hepatic fibrosis is a self-repair response of the liver to sustained injury. It is the abnormal structure and function of the liver caused by the excessive accumulation of ECM in the intercellular spaces caused by activation of HSCs and imbalance of synthesis and degradation of ECM. Chronic inflammation caused by sustained injury is the initiating factor of hepatic fibrosis[15]. When the pathogenic factors act on the body, a variety of injurious changes occur, but they also stimulate the body to produce defense, adaptation, compensation and other anti-injury responses. AnxA1 is a glucocorticoid regulatory protein that inhibits neutrophil apoptosis, enhances efferocytosis in macrophages, inhibits activation of phospholipase A2, downregulates expression of inflammatory arachidonic acid, and has a significant negative regulatory effect on several inflammatory cells and factors[22]. In this study, a mouse model of experimental liver fibrosis was established by intraperitoneal injection of 20%CCl4, and treatment or intervention was performed by knockout of AnxA1 gene, intraperitoneal injection of AnxA1-simulated peptide Ac2-26 and AnxA1 receptor inhibitor Boc2, to observe the effects of AnxA1 on liver fibrosis and explore its main targets. We found that AnxA1 protein and mRNA levels in the liver fibrosis model group were higher than those in the normal control group, and were time-dependent. AnxA1 began to increase at 4 wk, peaked at 8 wk, and then began to decline, consistent with the severity of liver fibrosis in mice, suggesting that AnxA1 is associated with the onset of liver fibrosis. Further studies showed that AnxA1 inhibited fibrosis. Firstly, gross liver specimens revealed that the liver surface roughness and nodulation of mice in the AnxA1-/- model group were more severe than those in the wild-type model group, while the lesions in the Ac2-26 group were significantly reduced after treatment, suggesting that Ac2-26 alleviated liver damage. Secondly, HE staining showed that compared with the wild-type model group, liver damage and inflammatory cell infiltration were further aggravated in the AnxA1-/- model group, suggesting serious liver damage, which was significantly reduced after treatment with Ac2-26. In addition, Masson staining indicated collagen deposition in the portal area in the wild-type model group, CCl4 induced more significant collagen deposition in the liver tissue of the AnxA1-/- model group, with a wide area, especially at 8 wk, when large amounts of collagen surrounded the liver tissue to form pseudolobules rather than concentrating in the portal area. Collagen deposition in liver tissue of the AnxA1-/- model group progressed significantly, and was significantly improved in the group treated with Ac2-26. AnxA1 inhibited liver inflammation and collagen deposition, and relieved liver fibrosis. AnxA1 may play an important role in the occurrence and development of liver fibrosis. While Boc2 blocked the anti-inflammatory and antifibrotic effects of Ac2-26, AnxA1 may have participated in the pathogenesis of liver fibrosis by targeting FPR. Chronic liver disease is progressive, in which inflammation and fibrosis form a vicious cycle, and eventually the excess deposition of ECM, especially type I collagen, makes the lesions difficult to reverse[19,20]. The results of our study showed that the collagen I concentration in liver tissues of mice in the CCl4-induced model group was significantly increased, and collagen I content in the AnxA1-/- model group was higher than that in the wild-type model group, and Boc2 reversed the therapeutic effect of Ac2-26 by reducing the collagen I concentration in liver tissues. High collagen I expression was maintained in the CCl4-induced model group. It was verified that AnxA1 inhibited liver collagen deposition and relieved liver fibrosis through FPR. Therefore, AnxA1 has a protective effect on hepatic fibrosis.
There have been few studies on the effects of AnxA1 on liver fibrosis. The possible protective mechanism of AnxA1 on liver fibrosis is discussed. HSCs play an important role in the formation of liver fibrosis[19,20], and activation of HSCs is the main cause of deposition of fibrous collagen (types I, III and IV) and other stroma-like proteins in chronic liver disease[23,24], and α-SMA is an activation marker of HSCs. The activation and development of HSCs into fibroblasts is accompanied by increased expression of α-SMA[25], and the degree of HSC activation can be determined by detecting the expression level of α-SMA. In this study, the expression of α-SMA was detected by western blotting and qPCR. The expression of α-SMA in liver tissue of mice in the CCl4-induced model group was higher than that in the control group, and the activation of HSCs induced by CCl4 in AnxA1-/- mice was higher than that in wild-type mice. Supplementation of exogenous Ac2-26 significantly inhibited the expression of α-SMA, and Boc2 blocked the inhibitory effect of Ac2-26. It is suggested that Ac2-26 inhibits HSC activation, which may be one of the key factors in inhibiting liver fibrosis by AnxA1, in which FPR receptors are involved.
HSCs are the main receivers of inflammatory signals and the main promoters of liver fibrosis. Hepatocyte injury triggers an inflammatory response, releases various cytokines and inflammatory factors, activates HSCs, and causes secretion and deposition of ECM, leading to liver fibrosis[26,27]. Immune cells, especially Kupffer cells and macrophages in the liver, are key regulators of inflammation[28]. Kupffer cells are intrinsic macrophages in the liver, which play an important role in the elimination of and defense against bacteria and pathogenic substances from the gastrointestinal tract[29]. In the case of liver injury, Kupffer cells are activated by damage-associated pathogenic patterns or LPS, viral DNA, and other pathogen-associated molecular patterns, producing and releasing TGF-β1, IL-1β and IL-6 to activate HSCs, causing the secretion and deposition of ECM and leading to liver fibrosis[30-32]. Our results showed that hepatic fibrosis was accompanied by inflammatory infiltration and secretion of inflammatory cytokines TGF-β1, IL-1β and IL-6, which increased with time, suggesting that hepatic inflammation is related to the severity of fibrosis. Exogenous Ac2-26 significantly reduced hepatic inflammatory cell infiltration and inflammatory factor expression in the CCl4-induced model group, while alleviating the formation of liver fibrosis, and Boc2 inhibited the anti-inflammatory effect of Ac2-26. AnxA1 may reduce fibrosis by inhibiting the inflammatory response in the liver, and its mechanism may be related to inhibition of HSC activation by targeting FPR to regulate macrophage function.
TGF-β1 is a key regulatory factor in the process of liver fibrosis[32], and CTGF, as its downstream factor, mediates TGF-β1, promoting ECM production and accumulation[33]. HSCs are the main source of CTGF, and blocking CTGF can significantly inhibit HSC activation and proliferation, and reduce tissue inhibitors of metalloproteinase, matrix metalloproteinase (MMP)2, MMP9 and type I collagen. Our results showed that increased expression of CTGF in the liver tissue of mice in the CCl4-induced model group was also inhibited by Ac2-26, suggesting that the inhibitory effect of AnxA1 on liver fibrosis is closely related to the expression of TGF-β1 and its downstream factor CTGF.
In order to verify the results of the animal experiments and to better investigate the mechanism of AnxA1 in liver fibrosis, we conducted in vitro cell experiments. Ac2-26 significantly inhibited LPS-induced activation of RAW264.7 cells and downregulated TGF-β1 and other inflammatory factors expressed in RAW264.7 cells after activation, and Boc2 reversed the above results. LPS induced HCs and HSCs to increase Anxa1 expression, but LPS induced RAW264.7 cells did not upregulate AnxA1 expression, and the activated HSCs that overexpressed SMA, collagen, and CTGF were inhibited by exogenous Ac2-26. AnxA1 mediated by FPR may affect the function of macrophages through HC paracrine or HSC autocrine effects, control inflammatory responses, inhibit HSC activation, and reduce ECM deposition by negative feedback, so as to prevent liver fibrosis formation.
Recent studies have shown that the Wnt/β-catenin signaling pathway can promote liver fibrosis and play an important role in HSC proliferation, metastasis and ECM formation[34]. Inhibition of Wnt/β-catenin signaling can inhibit the proliferation of HSCs and induce their apoptosis, thus alleviating CCl4-induced liver fibrosis[34-36]. In addition, TGF-β1 activates the Wnt/β-catenin pathway by inhibiting the expression of Wnt/β-catenin inhibitors[36]. Our results suggest that Ac2-26 downregulates the expression of β-catenin in the Wnt signaling pathway in mouse liver fibrosis, and inhibits the expression of activated HSC β-catenin. Combined with previous findings, the therapeutic effects of AnxA1 may alleviate the development of liver fibrosis by inhibiting the Wnt/β-catenin pathway of HSCs.
AnxA1 may play an important role in the formation of liver fibrosis, including regulating macrophage function, reducing inflammation, inhibiting HSC activation, and improving collagen deposition. AnxA1 mediated by FPR receptors may regulate the activity of macrophages through HSC autocrine or paracrine functions, inhibit the Wnt/β-catenin pathway, and inhibit HSC activity. Although its further mechanism of action has not been clearly elucidated, our results suggest that AnxA1 has potential activity against hepatic fibrosis and can block the occurrence of fibrosis at multiple targets, which may provide a new option for liver fibrosis treatment.
Liver fibrosis is a pathological change caused by chronic liver injury, and is a key link in the progression of chronic liver diseases to cirrhosis or liver cancer. How to prevent and reverse liver fibrosis is currently a hot and difficult research topic, and fully understanding and elucidating the pathogenesis of liver fibrosis is the foundation of prevention and treatment. At present, there is still a lack of safe and effective treatment for liver cirrhosis clinically. It is of great significance to deeply study the pathogenesis of liver fiber and prevent and treat it in order to reduce the incidence rate and mortality of liver cirrhosis.
Studies have shown that liver fibrosis is closely related to inflammation in chronic liver disease, and annexin (Anx)A1 has strong anti-inflammatory effects. Therefore, this study focuses on the pathogenesis of liver fibrosis and investigates the role of AnxA1 in liver fibrosis. Provide valuable information for antifibrosis therapy.
To investigate molecular and cellular mechanisms of liver fibrosis and evaluate the effect of AnxA1 in hepatic fibrosis.
Biomarkers as drug development tools, biomarker discovery and validation using a combination of in vitro and in vivo studies. A large and wide range of animal models and cell experiments can be used, and experiments can be repeated multiple times.
AnxA1/formylpeptide receptor (FPR) inhibits the activation of hepatic stellate cells by regulating macrophage function through the Wnt/β-catenin pathway in the CCl4-induced hepatic fibrosis mice.
AnxA1 may involve in the pathogenesis of liver fibrosis and as a potential therapeutic target.
This study investigates the mechanism of Anxa1 in liver fibrosis by using wild mice/Anxa1 knockout mice in combination with Anxa1 mimetic peptide and FPR receptor inhibitor to provide an experimental basis for the possible use of Anxa1 in the treatment of liver fibrosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Mohamed GA, Egypt; Nakade Y, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Caligiuri A, Gentilini A, Pastore M, Gitto S, Marra F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang S, Wang Y, Chen C, Li W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020;11:454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int. 2018;17:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology. 2016;150:181-193.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 568] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 7. | Lan T, Li C, Yang G, Sun Y, Zhuang L, Ou Y, Li H, Wang G, Kisseleva T, Brenner D, Guo J. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018;68:1070-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 8. | Cheng D, Chai J, Wang H, Fu L, Peng S, Ni X. Hepatic macrophages: Key players in the development and progression of liver fibrosis. Liver Int. 2021;41:2279-2294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in Liver Fibrosis. Semin Liver Dis. 2017;37:119-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Gan C, Cai Q, Tang C, Gao J. Inflammasomes and Pyroptosis of Liver Cells in Liver Fibrosis. Front Immunol. 2022;13:896473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 12. | Senchenkova EY, Ansari J, Becker F, Vital SA, Al-Yafeai Z, Sparkenbaugh EM, Pawlinski R, Stokes KY, Carroll JL, Dragoi AM, Qin CX, Ritchie RH, Sun H, Cuellar-Saenz HH, Rubinstein MR, Han YW, Orr AW, Perretti M, Granger DN, Gavins FNE. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation. 2019;140:319-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Xu X, Gao W, Li L, Hao J, Yang B, Wang T, Bai X, Li F, Ren H, Zhang M, Zhang L, Wang J, Wang D, Zhang J, Jiao L. Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway. J Neuroinflammation. 2021;18:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Vecchi L, Alves Pereira Zóia M, Goss Santos T, de Oliveira Beserra A, Colaço Ramos CM, França Matias Colombo B, Paiva Maia YC, Piana de Andrade V, Teixeira Soares Mota S, Gonçalves de Araújo T, Van Petten de Vasconcelos Azevedo F, Soares FA, Oliani SM, Goulart LR. Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo. Biochim Biophys Acta Mol Cell Res. 2018;1865:1368-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Neymeyer H, Labes R, Reverte V, Saez F, Stroh T, Dathe C, Hohberger S, Zeisberg M, Müller GA, Salazar J, Bachmann S, Paliege A. Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol (Oxf). 2015;215:144-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Lai T, Li Y, Mai Z, Wen X, Lv Y, Xie Z, Lv Q, Chen M, Wu D, Wu B. Annexin A1 is elevated in patients with COPD and affects lung fibroblast function. Int J Chron Obstruct Pulmon Dis. 2018;13:473-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget. 2017;8:90579-90604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Yu FJ, Dong PH, Fan XF, Lin Z, Chen YP, Li J. Down-regulation of angiotensin II by shRNA reduces collagen synthesis in hepatic stellate cells. Int J Mol Med. 2010;25:801-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Katoh M. Multilayered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/βcatenin signaling activation (Review). Int J Mol Med. 2018;42:713-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Zhang C, Zhang Y, Zhang W, Tong H, Li S, Yan Y. WISP1 promotes bovine MDSC differentiation via recruitment of ANXA1 for the regulation of the TGF-β signalling pathway. Mol Cell Biochem. 2020;470:215-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Fan JH, Feng GG, Huang L, Tang GD, Jiang HX, Xu J. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J Gastroenterol. 2014;20:4963-4971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Zharkova O, Salamah MF, Babak MV, Rajan E, Lim LHK, Andrade F, Gil CD, Oliani SM, Moraes LA, Vaiyapuri S. Deletion of Annexin A1 in Mice Upregulates the Expression of Its Receptor, Fpr2/3, and Reactivity to the AnxA1 Mimetic Peptide in Platelets. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 23. | Hall A, Cotoi C, Luong TV, Watkins J, Bhathal P, Quaglia A. Collagen and elastic fibres in acute and chronic liver injury. Sci Rep. 2021;11:14569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Rong X, Yang Y, Zhang G, Zhang H, Li C, Wang Y. Antler stem cells as a novel stem cell source for reducing liver fibrosis. Cell Tissue Res. 2020;379:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wang R, Zhang D, Tang D, Sun K, Peng J, Zhu W, Yin S, Wu Y. Amygdalin inhibits TGFβ1-induced activation of hepatic stellate cells (HSCs) in vitro and CCl(4)-induced hepatic fibrosis in rats in vivo. Int Immunopharmacol. 2021;90:107151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Delgado ME, Cárdenas BI, Farran N, Fernandez M. Metabolic Reprogramming of Liver Fibrosis. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Papachristoforou E, Ramachandran P. Macrophages as key regulators of liver health and disease. Int Rev Cell Mol Biol. 2022;368:143-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Seo HY, Lee SH, Han E, Hwang JS, Han S, Kim MK, Jang BK. Evogliptin Directly Inhibits Inflammatory and Fibrotic Signaling in Isolated Liver Cells. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, Fu Q, Maehara O, Suda G, Sakamoto N. Extracellular Vesicles from Amnion-Derived Mesenchymal Stem Cells Ameliorate Hepatic Inflammation and Fibrosis in Rats. Stem Cells Int. 2018;2018:3212643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Xiang DM, Sun W, Ning BF, Zhou TF, Li XF, Zhong W, Cheng Z, Xia MY, Wang X, Deng X, Wang W, Li HY, Cui XL, Li SC, Wu B, Xie WF, Wang HY, Ding J. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67:1704-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, Varol C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 33. | Trampuž SR, van Riet S, Nordling Å, Ingelman-Sundberg M. The Role of CTGF in Liver Fibrosis Induced in 3D Human Liver Spheroids. Cells. 2023;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 34. | Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 35. | Lee IH, Im E, Lee HJ, Sim DY, Lee JH, Jung JH, Park JE, Shim BS, Kim SH. Apoptotic and antihepatofibrotic effect of honokiol via activation of GSK3β and suppression of Wnt/β-catenin pathway in hepatic stellate cells. Phytother Res. 2021;35:452-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | El-Fadaly AA, Afifi NA, El-Eraky W, Salama A, Abdelhameed MF, El-Rahman SSA, Ramadan A. Fisetin alleviates thioacetamide-induced hepatic fibrosis in rats by inhibiting Wnt/β-catenin signaling pathway. Immunopharmacol Immunotoxicol. 2022;44:355-366. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |