Copyright
©The Author(s) 2023.
World J Gastroenterol. May 28, 2023; 29(20): 3048-3065
Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3048
Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3048
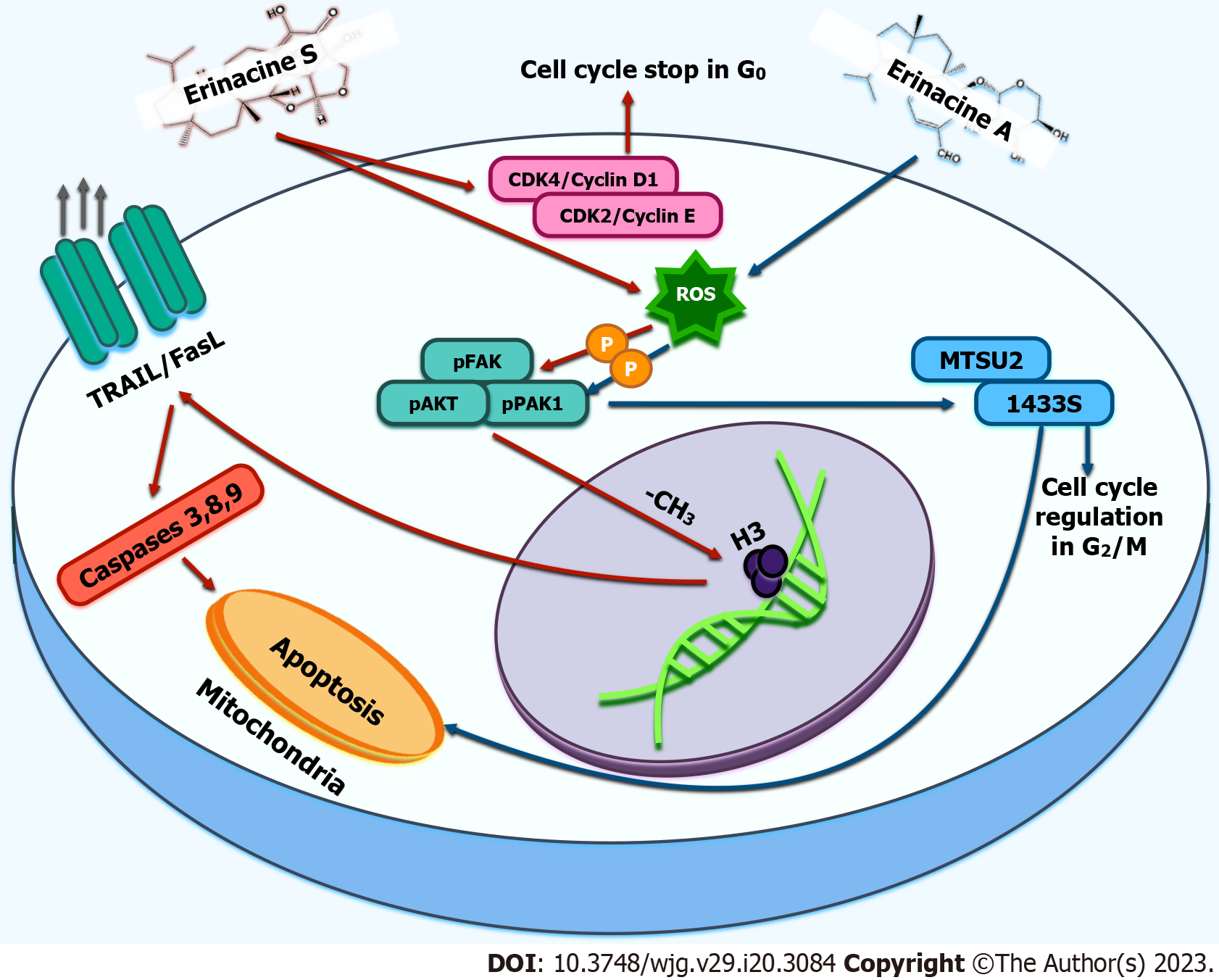
Figure 2 Main antineoplastic molecular mechanisms of erinacines S and A from Hericiumerinaceus in gastric cancer cell models.
Erinacine S (in AGS gastric cancer cells) can activate a pathway (red arrows) with reactive oxygen species (ROS) mediated phosphorylation of focal adhesion kinase (FAK)/protein kinase B (also known as AKT)/p21-activated kinase 1 (PAK1). Subsequently, by trimethylation of histone H3, the latter pathway can induce the increased expression of TNF-related apoptosis-inducing ligand T and Fas ligand receptors by the cancer cell with the subsequent activation of apoptosis by initiating caspases 3, 8, and 9. Erinacine A (in TSGH9201 gastric cancer cells), once activated (blue arrows), the FAK/AKT/PAK1 pathway, in a similar manner as previously described, upregulates microtubule-associated scaffold protein 2/14-3-3 protein sigma proteins with subsequent activation of caspases-mediated apoptosis. In addition, erinacines can also modulate cell cycle regulation by preventing cell cycle continuation through the blockade at checkpoints, i.e., blocking cyclin-dependent kinases. ROS: Reactive oxygen species; FAK: Focal adhesion kinase; PAK: Activated kinase 1; MTUS2: Microtubule-associated scaffold protein 2; TRAIL: TNF-related apoptosis-inducing ligand T; Fas-L: Fas ligand; CDKs: Cyclin-dependent kinases; 1433S: 14-3-3 protein sigma.
- Citation: Gravina AG, Pellegrino R, Auletta S, Palladino G, Brandimarte G, D’Onofrio R, Arboretto G, Imperio G, Ventura A, Cipullo M, Romano M, Federico A. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J Gastroenterol 2023; 29(20): 3048-3065
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3048.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3048