Copyright
©The Author(s) 2023.
World J Gastroenterol. Mar 28, 2023; 29(12): 1779-1794
Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1779
Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1779
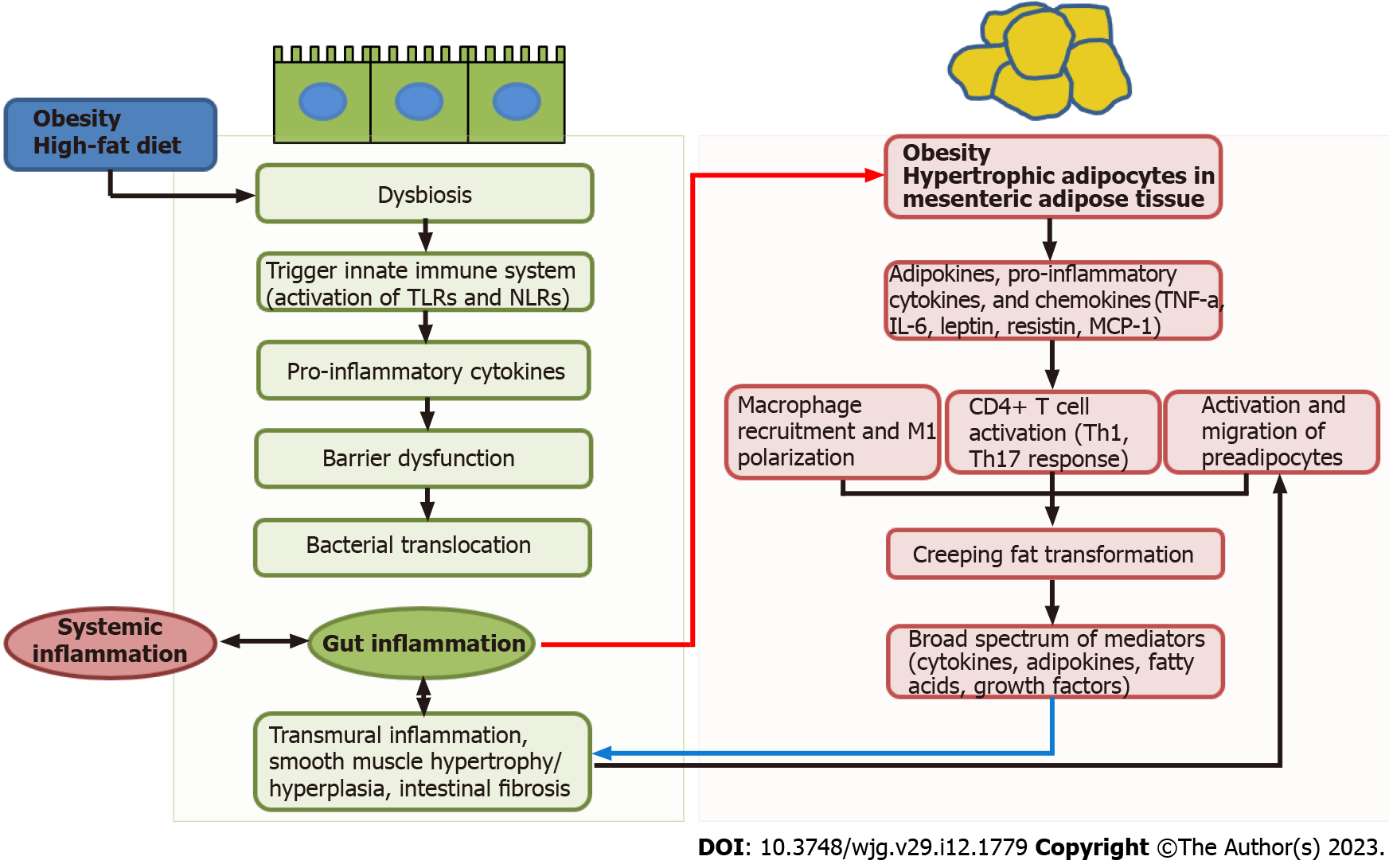
Figure 1 High fat or Western diets decreases the diversity of the gut microbiota (dysbiosis), leading to the reduction of anti-inflammatory bacterial species or metabolites.
These changes can trigger innate immune system by activation of pattern recognition receptors such as Toll-like receptors or Nod-like receptors on intestinal epithelial cells or innate immune cells. Pro-inflammatory cytokines secreted from innate immune system induces barrier dysfunction and subsequent bacterial translocation. Low grade chronic inflammation in gut and bacterial translocation to mesenteric adipose tissue leads to the adipocyte hypertrophy (red arrow). The hypertrophic adipocytes release adipokines, pro-inflammatory cytokines, and chemokines such as tumor necrosis factor alpha, interleukin-6, leptin, resistin, and monocyte chemoattractant protein-1. These pro-inflammatory mediators can induce macrophage recruitment and polarization and CD4+ T cell activation into T helper 1 (Th1) and Th17 cells. Finally, this process can drive the hypertrophic mesenteric fat wrapping around the inflamed bowel, creeping fat. Creeping fat secretes a broad spectrum of mediators, and induce smooth muscle hypertrophy/hyperplasia, transmural inflammation, intestinal fibrosis. TLR: Toll-like receptor; NLR: Nod-like receptor; TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin; MCP-1: Monocyte chemoattractant protein 1; Th: T helper.
- Citation: Kim JH, Oh CM, Yoo JH. Obesity and novel management of inflammatory bowel disease. World J Gastroenterol 2023; 29(12): 1779-1794
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1779