Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4328
Peer-review started: March 11, 2022
First decision: May 10, 2022
Revised: May 12, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: August 21, 2022
Bile acids play an important role in the amelioration of type 2 diabetes following duodenal-jejunal bypass (DJB). Serum bile acids are elevated postoperatively. However, the clinical relevance is not known. Bile acids in the peripheral circulation reflect the amount of bile acids in the gut. Therefore, a further investigation of luminal bile acids following DJB is of great significance.
To investigate changes of luminal bile acids following DJB.
Salicylhydroxamic acid (SHAM), DJB, and DJB with oral chenodeoxycholic acid (CDCA) supplementation were performed in a high-fat-diet/streptozotocin-induced diabetic rat model. Body weight, energy intake, oral glucose tolerance test, luminal bile acids, serum ceramides and intestinal ceramide synthesis were analyzed at week 12 postoperatively.
Compared to SHAM, DJB achieved rapid and durable improvement in glucose tolerance and led to increased total luminal bile acid concentrations with preferentially increased proportion of farnesoid X receptor (FXR) - inhibitory bile acids within the common limb. Intestinal ceramide synthesis was repressed with decreased serum ceramides, and this phenomenon could be partially antagonized by luminal supplementation of FXR activating bile acid CDCA.
DJB significantly changes luminal bile acid composition with increased proportion FXR-inhibitory bile acids and reduces serum ceramide levels. There observations suggest a novel mechanism of bile acids in metabolic regulation after DJB.
Core Tip: Bile acids play an important role in the amelioration of type 2 diabetes following duodenal-jejunal bypass (DJB), and are elevated significantly in the serum postoperatively. Bile acids in the peripheral circulation reflect the amount of bile acids in the gut. Therefore, a further investigation of luminal bile acids following DJB is of great significance. Here we performed DJB in a high-fat diet/streptozotocin-induced diabetic rat model and demonstrated that DJB achieved rapid and durable improvement in glucose tolerance and led to increased total luminal bile acid concentrations with preferentially increased proportion of farnesoid X receptor (FXR) - inhibitory bile acids within the common limb. Intestinal ceramide synthesis was repressed with decreased serum ceramides, and this phenomenon could be partially antagonized by luminal supplementation of FXR activating bile acid (chenodeoxycholic acid). There observations suggest a novel mechanism of bile acids in metabolic regulation after DJB.
- Citation: Cheng ZQ, Liu TM, Ren PF, Chen C, Wang YL, Dai Y, Zhang X. Duodenal-jejunal bypass reduces serum ceramides via inhibiting intestinal bile acid-farnesoid X receptor pathway. World J Gastroenterol 2022; 28(31): 4328-4337
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4328
Duodenal-jejunal bypass (DJB) can induce rapid and durable amelioration of type 2 diabetes mellitus[1-3]. The underlying mechanisms remain incompletely understood. Our previous research has proved that bile acids play an important role in the amelioration of type 2 diabetes following DJB[4], and found that serum taurine-conjugated bile acids are preferentially elevated postoperatively[5]. However, the clinical relevance of the specific alterations of serum bile acids is still not known. Bile acids in the peripheral circulation reflect the amount of bile acids that could not be totally reabsorbed by hepatocytes during the enterohepatic circulation[6]. Therefore, the alterations of serum bile acids might be a secondary change of the bile acids within the gut, and a further investigation of luminal bile acids following DJB is of great significance.
Bile acids are traditionally known as lipid absorption-facilitating agents. It was not until recent years that the role of bile acids as signaling molecules in modulating metabolism has be unveiled. The intestinal lumina, where bile acid concentrations are high, is the main place for bile acid signaling. Two major receptors, including Takeda G-protein-coupled receptor 5 (TGR5) and nuclear farnesoid X receptor (FXR) are responsible for luminal bile acid sensing. TGR5 expression is detected in a variety of enteroendocrine cells and acute exposure of TGR5 to luminal bile acids lead to significant secretion of glucagon-like peptide 1 (GLP-1), which is a vital hormone for maintaining normal incretin effect in type 2 diabetes[7,8]. The interaction between bile acids and FXR is more complicated, as different subtypes of bile acids have distinct effect on the downstream pathway of FXR[9]. Chenodeoxycholic acid (CDCA) represents the most potent FXR stimulator while ursodeoxycholic acid (UDCA) and β-muricholic acid (βMCA) are FXR inhibitors[9-11]. Therefore, the net effect of luminal bile acids on FXR depends on the proportion of FXR-stimulating bile acids rather than the total amount of bile acids.
Intestinal FXR could affect lipid metabolism and this process is closely related to ceramide synthesis. Intestine-selective FXR inhibition downregulates the expression of ceramide synthesis-related genes sphingomyelin phosphodiesterase 3 (Smpd3) and serine palmitoyltransferase long chain base subunit 2 (Sptlc2), resulting in decreased concentrations of ceramides within the small intestine, portal system and peripheral circulation[12]. Decreased ceramides inhibit the expression of sterol regulatory element binding protein-1 (SREBP-1) in the liver[12], which is a key enzyme in the process of hepatic fat accumulation. Coincidentally, the changes in lipid metabolism after intestine-selective FXR inhibition is similar to the changes following DJB that hepatic fat accumulation is alleviated and the key transcriptional regulators and enzymes involved in hepatic de novo lipogenesis are downregulated[13]. Therefore, we hypothesized that the net effect of luminal bile acids on intestinal FXR might be inhibitory after DJB which leads to decreased ceramide synthesis. To test this hypothesis, we measure the changes of individual luminal bile acid and ceramide concentrations within the enterohepatic circulation after DJB in a high-fat diet (HFD)/streptozotocin (STZ)-induced diabetic rat model.
Eight-week-old male Wistar rats (220 g on average, purchased from Huafukang Biotech, China) were individually housed with a 12 h light/dark cycle under constant temperature (24-26 °C) and humidity (50%-70%). All rats were fed with HFD (42% carbohydrate, 18% protein and 40% fat, as a total percentage of calories, Huafukang Biotech, China) with no restriction to tap water for 1 mo to induce insulin resistance. After fasting for 12 h, 30 mg/kg STZ (Sigma Aldrich, United States) dissolved in sodium citrate buffer (pH 4.2) was injected into the peritoneal cavity. Random blood glucose concentrations were measured with a glucometer (Roche Diagnostics, Germany) from tail veins 72 h later. Thirty rats with random blood glucose ≥ 16.7 mmol/L were considered diabetic and were matched into salicylhydroxamic acid (SHAM) group (n = 10), DJB group (n = 10) and DJB + CDCA group (n = 10). One month later, surgery was performed as we previously reported[5]. Body weight and calorie intake were recorded daily. All rats were sacrificed after 12 h fasting at week 12 postoperatively. All procedures involving animals were reviewed and approved by the Ethics Committee on Animal Experiment of Shandong University Qilu Hospital.
Without fasting, rats in the DJB + CDCA group were administrated with CDCA suspension (100 mg/kg suspended in 3 mL tap water) by intragastric gavage three times a week since week 5 postoperatively. The gavage procedure ceased after week 11.
At week 4 and week 12, after 12 h fasting, the rats were administrated with 20% of glucose (1 g/kg) by intragastric gavage. Blood glucose concentrations were measured at t = 0, 10, 30, 60 and 120 min.
Peripheral blood samples were collected from the retrobulbar venous plexus after 12 h fasting before sacrificing. Portal venous blood samples were collected directly from the portal vein. After centrifugation, the supernatant was stored at -80 °C until analysis.
Three independent intestinal segments (3 cm each) was excised at the proximal, medium and distal sites within the common limb, respectively, without prior flushing. The control intestinal segments from SHAM group were excised at the corresponding anatomic location. Total luminal bile acids from intestinal segments were extracted by 9 mL 50 % tert-butyl alcohol for 1 h at 37 °C. After centrifugation, the supernatant was collected for bile acid analysis. Total bile acids were measured by Roche Cobas 8000 system using enzyme cycling method and individual bile acid species was measured using high-pressure liquid chromatography coupled with tandem mass spectrometry as we previously described[5].
Small intestinal mucosa were flushed with saline and then scratched. Total protein was extracted by RIPA lysis buffer (KeyGEN, China) with protease and phosphatase inhibitor cocktail (KeyGEN, China) and was quantified using bicinchoninic acid protein assay kit (Beyotime, China). Equivalent amount of small intestinal mucosal protein were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (Bio-Rad, United States) and separated by electrophoresis. Then, proteins were transferred onto 0.45 μm polyvinylidene fluoride membranes (Millipore, Ireland). After being blocked in 5 % skim milk powder (Beyotime, China) for 2 h, the membranes were incubated with primary antibodies to Smpd3 (Abcam, United Kingdom) and Sptlc2 (Invitrogen, United States) overnight, followed by incubation in horseradish peroxidase-conjugated secondary antibodies (Proteintech, China) for 60 min. The protein bands were visualized by ECL solution (Millipore, United States) and their density was assessed with LI-COR Odyssey Imager (LI-COR Biosciences, United States).
The expression of smpd3 and sptlc2 in the small intestine was analyzed by real-ime quantitative polymerase chain reaction (RT-qPCR). RNA was isolated using Trizol reagent (Invitrogen, United States), and the concentration of RNA was measured using the NanoDrop spectrophotometer (NanoDrop Technologies, United States). RNA was then reverse transcribed into complementary DNA (cDNA) using a ReverTra Ace qPCR RT Kit (TOYOBO, Japan). Amplification of messenger RNA (mRNA) was carried out using SYBR Green Real-time PCR Master Mix Kit (TOYOBO, Japan) at a range of temperatures in a Roche Lightcycle 2.0 system (Roche, Switzerland). The housekeeping gene gapdh was used as an internal reference, and mRNA levels for each target were then calculated by the 2-ΔΔCt method. The primers were smpd3 (forward primer: 5’-ACTCGCTCGCAAGGCTCAATAATG-3’, reverse primer: 5’-CTGAAGCTGGCTGCACTGATGG-3’), sptlc2 (forward primer: 5’-CGCCTTCCTGAAGTGATTGCTCTC-3’, reverse primer: 5’-AGTCTACTACACCACGCCCTGAAG-3’), and gapdh (forward primer: 5’-ACCCTGTTGCTGTAGCCATATTC-3’; reverse primer: 5’-ACCCTGTTGCTGTAGCCATATTC-3’).
Ceramide concentrations peripheral circulation and the portal vein were measured as previously described using Ceramide LIPIDOMIX Mass Spec Standard (#330712X-1EA, Avanti, United States) as standards[14].
Data are mean ± SEM. Area under the curve (AUC) for oral glucose tolerance test (OGTT) was calculated by trapezoidal integration. Intergroup comparisons were performed by one-way analysis of variance followed by Bonferroni post hoc comparisons. P < 0.05 was considered statistically significant. All calculations were performed using SPSS version 25.0.
One rat in SHAM group died of intestinal obstruction. Two rats in DJB group and 2 rats in DJB + CDCA group died of anastomotic leak. At the end of the study, the number of rats alive in SHAM, DJB and DJB + CDCA groups were 9, 8 and 8, respectively.
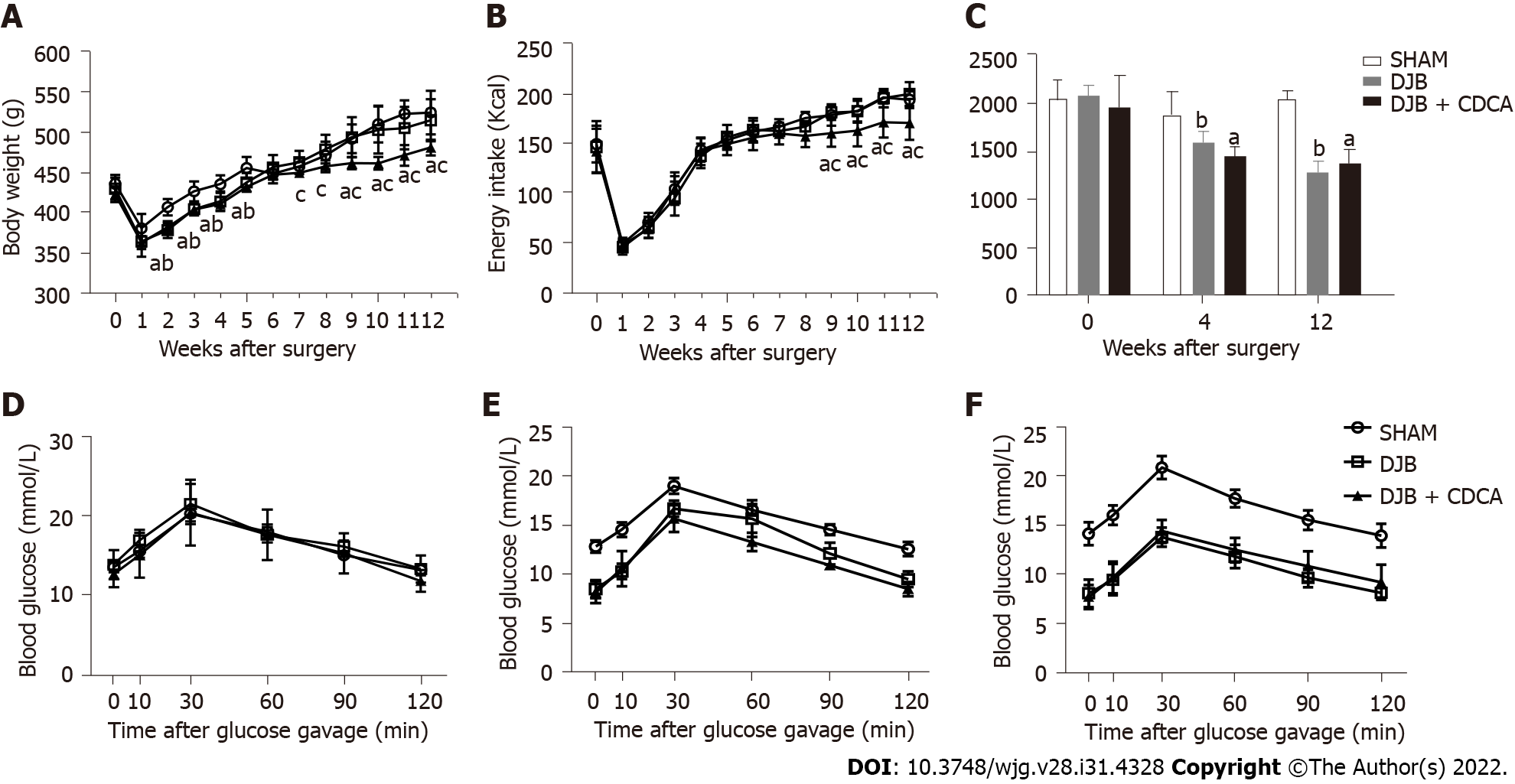
At baseline, both body weight and energy intake were comparable between three groups. After surgery, both body weight and energy intake decreased sharply in all three groups and increased gradually thereafter. At week 2-5 postoperatively, body weight of the rats in DJB and DJB + CDCA groups was significantly less than that in SHAM group (P < 0.05 all). At week 7-12 postoperatively, body weight of the rats in DJB + CDCA group was significantly less than that in DJB group (P < 0.05 all) (Figure 1A). At week 9-12 postoperatively, body weight of the rats in DJB + CDCA group was significantly less than that in SHAM group (P < 0.05 all). At week 9-12 postoperatively, energy intake of the rats in DJB + CDCA group was significantly less than that in SHAM and DJB groups (P < 0.05 all) (Figure 1B).
There was no difference in glucose tolerance between three groups at baseline based on AUCOGTT. At week 4 and 12, glucose tolerance was significantly improved with DJB and DJB + CDCA groups compared to SHAM group based on AUCOGTT (P < 0.05 each). There was no difference between DJB and DJB + CDCA groups, or between week 4 and week 12 within each group (Figures 1C-F).
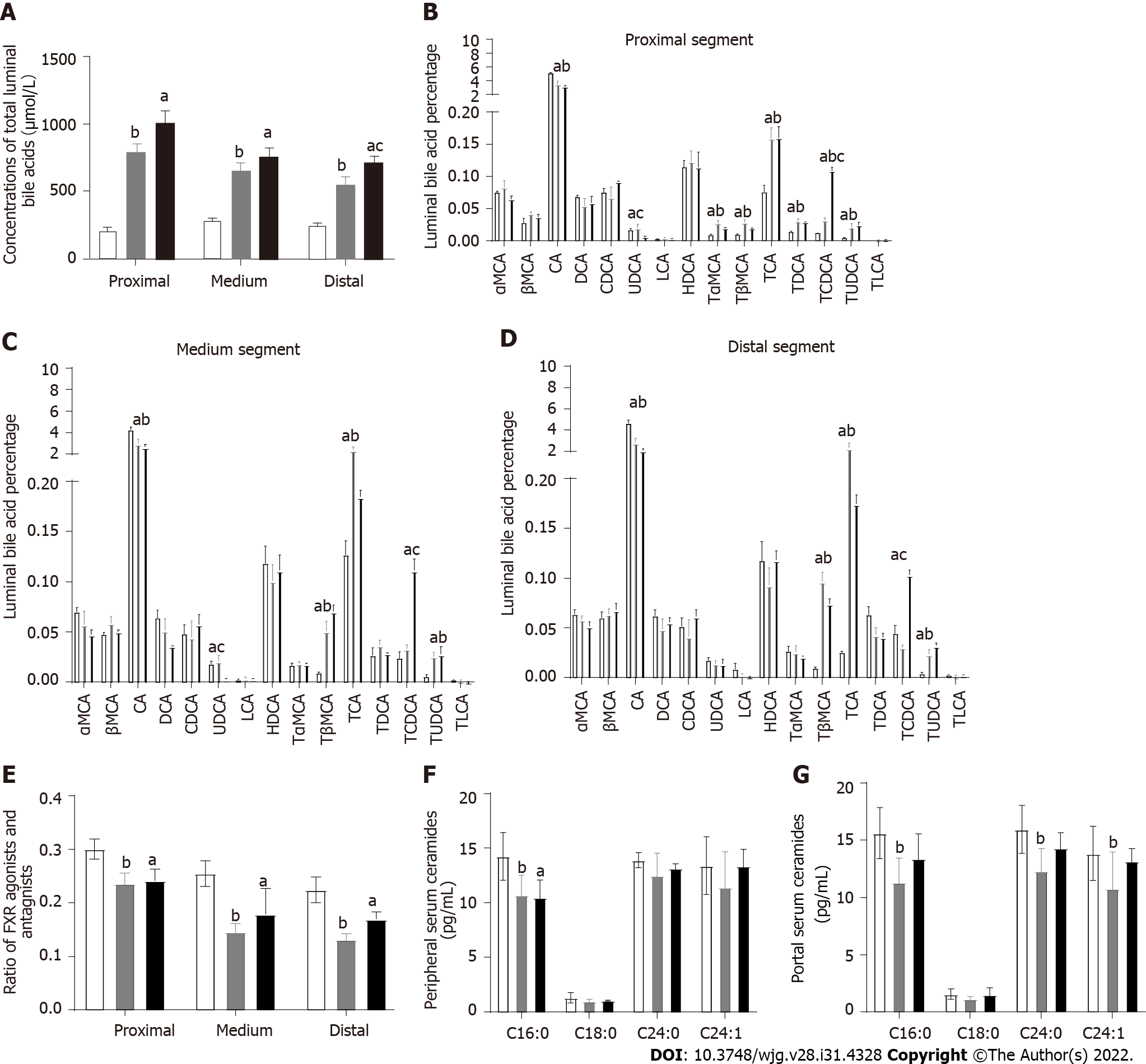
Total luminal bile acid concentrations were significantly less with SHAM group compared to both DJB and DJB + CDCA groups in either proximal, medium or distal segment in the common limb (P < 0.05 all). In the distal segment, luminal total bile acid concentrations were significantly greater with DJB + CDCA group than DJB group (P < 0.05), while this difference was not significant in the proximal or medium segment (Figure 2A).
In the proximal segment (Figure 2B), CA accounted for over 20% of the total luminal bile acids in all three groups, and was significantly greater with SHAM group than DJB and DJB + CDCA groups (P < 0.05). The percentages of α-muricholic acid (αMCA), βMCA, deoxycholic acid, CDCA, lithocholic acid (LCA), hyocholic acid (HDCA), and taurine-conjugated LCA (TLCA) were comparable between all three groups. The percentage of UDCA was less with DJB + CDCA group compared to SHAM and DJB groups (P < 0.05). The percentages of taurine-conjugated forms of αMCA, βMCA, CA, DCA, CDCA and UDCA were all greater with DJB and DJB + CDCA groups compared to SHAM group (P < 0.05). The percentage of TCDCA was greater with DJB + CDCA group than DJB group (P < 0.05).
In the medium segment (Figure 2C), the difference in TαMCA and TDCA between three groups was no longer significant compared to the proximal segment. The percentage of TβMCA increased to more than 5% in DJB and DJB + CDCA groups which was significantly greater than SHAM group (P < 0.05). The percentage of TCDCA was comparable between SHAM and DJB groups, which was significantly less than DJB + CDCA group (P < 0.05).
In the distal segment (Figure 2D), the percentage of TβMCA, TCA and TUDCA was significantly greater with DJB and DJB + CDCA groups compared to SHAM group (P < 0.05), without any difference between the former two groups. The percentage of CA was significantly less with DJB and DJB + CDCA groups compared to SHAM group (P < 0.05). There was no difference in the percentage of UDCA between three groups. The percentage of other individual bile acids was comparable to that in the medium segment. The ratio of FXR agonists (including CA, TCA, DCA, TDCA, CDCA, TCDCA, LCA, and TLCA) and antagonists (including αMCA, TαMCA, βMCA, TβMCA, UDCA, TUDCA and HDCA) in DJB and DJB + CDCA groups was decreased compared to SHAM group in either proximal, medium or distal segment (P < 0.05). No difference was observed between DJB group and DJB + CDCA group (Figure 2E).
At week 12, in the peripheral circulation, serum C16:0 ceramide concentrations were lower with DJB and DJB + CDCA groups compared to SHAM group (P < 0.05); no difference in C18:0, C24:0 or C24:1 ceramides between three groups (Figure 2F). In the portal vein, serum C16:0, C24:0 and C24:1 ceramide concentrations were lower with DJB group compared to SHAM group (P < 0.05); there was no difference in either ceramide species between DJB and DJB + CDCA groups or between DJB + CDCA and SHAM groups (Figure 2G).
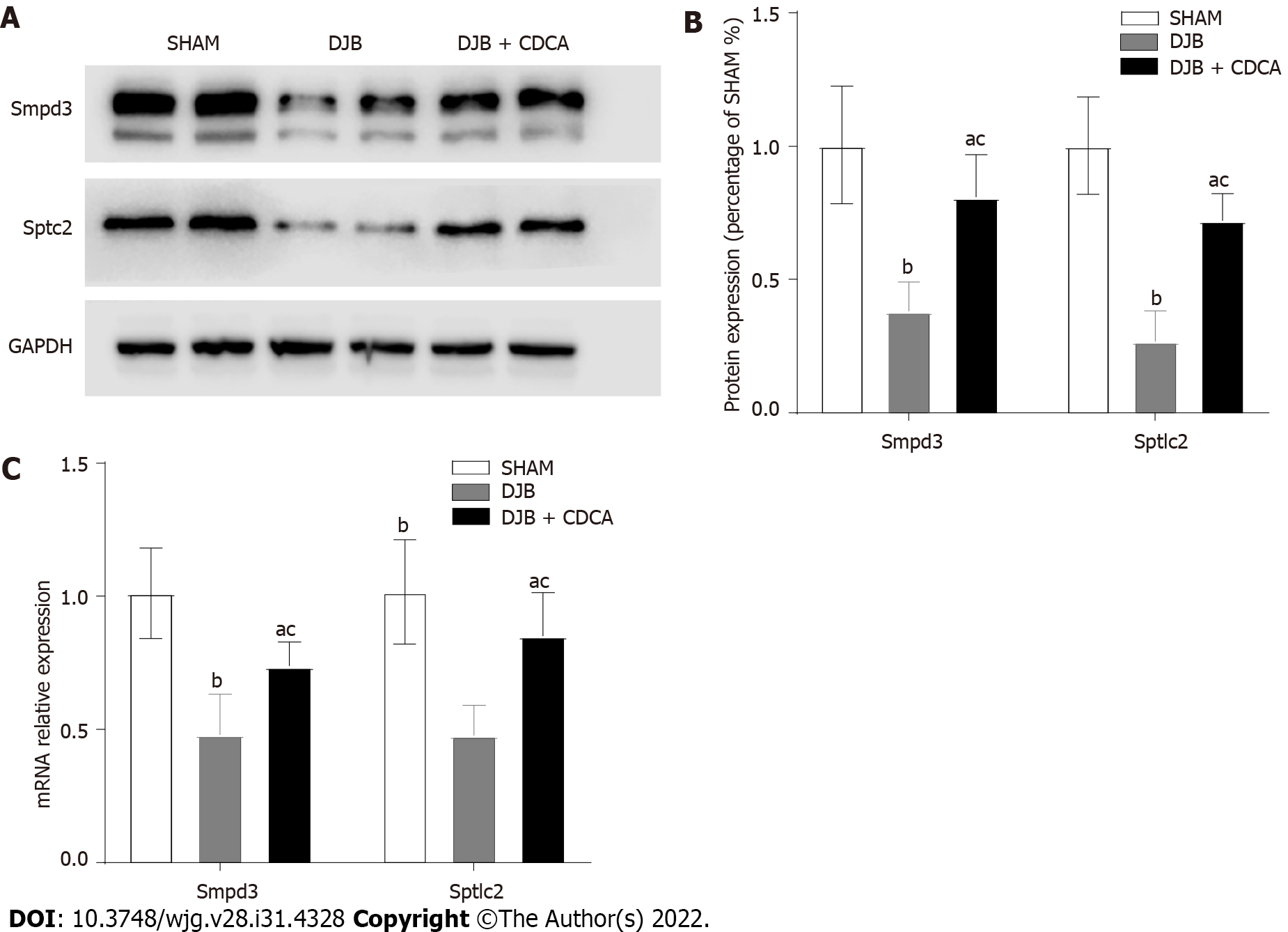
At week 12, the expression of Smpd3 and Sptlc2 were lower with DJB and DJB + CDCA groups than SHAM group at both protein and mRNA levels (P < 0.05). Compared to DJB + CDCA group, the expression of Smpd3 and Sptlc2 with DJB group were even lower at both protein and mRNA levels (P < 0.05) (Figures 3A, 3B and 3C).
In the present study, we performed SHAM, DJB and DJB + CDCA procedures in a HFD/STZ induced diabetic rat model, and for the first time, demonstrated the changes of luminal individual bile acids in the distal small intestine. We also found that the subsequent changes of the bile acids within the distal common limb after DJB elicited inhibitory effect on regional ceramide synthesis and this phenomenon could be partially antagonized by luminal supplementation of FXR activating bile acid CDCA.
DJB was initially designed to investigate the weight loss independent mechanisms of bariatric surgery[3]. This procedure has no effect on weight loss but could induce fast and sustainable amelioration of type 2 diabetes[4]. Consistent with other bariatric procedures[15,16], serum bile acid concentrations were also increased following DJB, and this phenomenon is clearly related to reconstruction of the gastrointestinal tract[5]. Via the biliopancreatic limb, bile acids contact the distal small intestine, where luminal bile acid reabsorption mainly occurs, more rapidly and lead to early and increased reabsorption of luminal bile acids in the small intestine. A recent study has proved that in the biliopancreatic limb, increased reabsorption of luminal bile acids has already commenced as a result of highly concentrated bile acids as well as lack of lipids[17]. While in the alimentary limb, only trace amount of luminal bile acids could be detected[4]. Decreased luminal bile acids lead to marked luminal sodium insufficiency, and hence, intestinal uptake of glucose in the alimentary limb is significantly decreased[18], which represents another mechanism in controlling postprandial glucose excursion. The common limb is where food and bile mix up and the major place for intestinal FXR expression. Therefore, we concentrated on bile acid milieu within the common limb rather than the biliopancreatic or alimentary limb because the common limb is the place mostly close to physiological conditions.
To our knowledge, the present study is the first study reporting luminal bile acid changes after DJB. Most clinical and animal studies concentrated on serum or fecal bile acids, as the intestinal lumen is deep inside and in vivo study of luminal contents, particularly in the small intestine, is technically difficult and ethically challenging. Consistent with our previous findings of serum bile acid changes after DJB, the total amount of luminal bile acids and the proportion of FXR-inhibitory bile acids were both increased. These specific changes have at least two clinical relevance. First, concentrated luminal bile acids stimulate TRG5 on the surface of enteroendocrine cells and leads to potentiated GLP-1 secretion[4]; second, increased proportion of FXR-inhibitory bile acids has inhibited expression of FXR downstream pathways and reduced biosynthesis of intestinal-derived ceramides[12]. Compared to TGR5, FXR appears to be a more important and complicated receptor in metabolic regulation; in the absence of FXR, the ability of bariatric surgery to reduce body weight and improve glucose tolerance is substantially reduced while these metabolic benefits are largely preserved when TGR5 is deficient[19-20]. Whole body FXR knock-out mice were associated with elevated serum triglycerides, cholesterols, free fatty acids and severe liver fat accumulation, but were protected from diet- or genetically- induced obesity[21]. In contrast, liver-specific FXR knock-out mice were not protected from diet-induced obesity and insulin resistance[22], suggesting the distinct role of hepatic and intestinal FXR activation in improving glucose tolerance and insulin resistance. In the small intestine, the role of FXR is controversial. After bariatric surgery, increased serum fibroblast growth factor 19 (FGF19) concentrations have been thought to play a role in the remission of human diabetes[15]. And intestinal FXR activation by luminal bile acids has been thought as a major source of increased serum FGF19. However, no direct evidence was available to confirm the state of intestinal FXR activation, and other tissues may also be sources of FGF19. In contrast, more studies support that intestinal FXR activation would damage metabolic homeostasis by reducing energy expenditure and impairing glucose tolerance[12,14,23]. To investigate the direct influence of bile acids on intestinal FXR, bile diversion procedure was reported by three separate studies[4,24,25], including one from our group[4]. Surprisingly, all three studies showed activated intestinal FXR in response to direct bile acid stimulation. However, in contrast, in DJB, the effect of bile acids on intestinal FXR within the common limb was inhibitory. The discrepancy suggests the biliopancreatic limb may have altered the luminal bile acid composition by premature bile acid reabsorption.
Consistent with our hypothesis, both ceramides in the portal vein and in the peripheral circulation were decreased in response to increased proportion of luminal FXR-inhibitory bile acids. Ceramides are signaling molecules and are associated with obesity and insulin resistance at high concentrations[26]. Decreased ceramides inhibits the expression of SREBP-1 in the liver and alleviates hepatic fat accumulation, thus increasing hepatic insulin sensitivity[12]. Our previous study found that DJB suppressed hepatic de novo lipogenesis and alleviates liver fat accumulation by inhibiting SREBP-1. However, the mechanisms underlying were unknown. Based on results from the present study, we have unveiled at least one mechanism accounting for alleviated hepatic fat accumulation. Therefore, manipulation of luminal bile acid composition towards FXR-inhibitory trend may have metabolic beneficial effects.
The present study has several limitations. First, bile acids are mixture with a variety of individual bile acids. As bile acids are mainly conjugated with taurine in rodents[27], we only tested luminal unconjugated and taurine-conjugated bile acids. Second, CDCA is not dissolved in water and we used CDCA suspension for gavage, which may have compromised CDCA absorption to a certain degree. Third, the results from the present study were based on a diabetic rat model and should be interpreted with caution due to species gap.
In conclusion, DJB significantly changes luminal bile acid composition with increased proportion of FXR-inhibitory bile acids and reduce serum ceramide levels. There observations suggest a novel mechanism of bile acids in metabolic regulation after DJB.
Bile acids have proved to be signaling molecules in mediating metabolic benefits and circulating bile acids were found to be increased following duodenal-jejunal bypass (DJB). However, whether and how the bile acids take effect in the amelioration of metabolic disorders after DJB remain unknown.
Circulating bile acids reflect the amount of bile acids within the gut. It has been proved that luminal bile acids take effect via Takeda G-protein-coupled receptor 5 and nuclear farnesoid X receptor (FXR). However, different subtypes of bile acids have distinct effect on the downstream pathway of FXR and hence, a further investigation of luminal bile acids after DJB is of great significance.
To investigate changes and mechanisms of luminal bile acids in mediating metabolic benefits following DJB.
Salicylhydroxamic acid (SHAM), DJB, and DJB with oral chenodeoxycholic acid (CDCA) supplementation were performed in a high-fat-diet/streptozotocin-induced diabetic rat model. Body weight, energy intake, oral glucose tolerance test, luminal bile acids, serum ceramides and intestinal ceramide synthesis were analyzed at week 12 postoperatively.
Compared to SHAM, DJB achieved rapid and durable improvement in glucose tolerance and led to increased total luminal bile acid concentrations with preferentially increased proportion of FXR - inhibitory bile acids within the common limb. Intestinal ceramide synthesis was repressed with decreased serum ceramides, and this phenomenon could be partially antagonized by luminal supplementation of FXR activating bile acid CDCA.
DJB significantly changes luminal bile acid composition with increased proportion FXR-inhibitory bile acids and reduces serum ceramide levels. There observations suggest a novel mechanism of bile acids in metabolic regulation after DJB.
Mechanisms of bile acids in mediating metabolic benefits after bariatric surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Becchetti C, Switzerland; Cereatti F, Italy; Pazos F, Spain S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Cohen R, Caravatto PP, Correa JL, Noujaim P, Petry TZ, Salles JE, Schiavon CA. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg Obes Relat Dis. 2012;8:375-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Ramos AC, Galvão Neto MP, de Souza YM, Galvão M, Murakami AH, Silva AC, Canseco EG, Santamaría R, Zambrano TA. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI<30 kg/m2 (LBMI). Obes Surg. 2009;19:307-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 608] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Liu T, Wang Y, Zhong M, Zhang G, Liu S, Wu T, Rayner CK, Hu S. Comparative Effects of Bile Diversion and Duodenal-Jejunal Bypass on Glucose and Lipid Metabolism in Male Diabetic Rats. Obes Surg. 2016;26:1565-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Wang Y, Zhong M, Liu T, Han H, Zhang G, Liu S, Wei M, Wu Q, Hu S. Duodenal-Jejunal Bypass Preferentially Elevates Serum Taurine-Conjugated Bile Acids and Alters Gut Microbiota in a Diabetic Rat Model. Obes Surg. 2016;26:1890-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 163] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Goldspink DA, Lu VB, Billing LJ, Larraufie P, Tolhurst G, Gribble FM, Reimann F. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol Metab. 2018;7:90-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, Rayner CK. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab. 2013;15:474-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2012] [Cited by in F6Publishing: 1993] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 10. | Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, Hoesel B, Fauler G, Stojakovic T, Einarsson C, Marschall HU, Trauner M. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398-1404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1367] [Cited by in F6Publishing: 1461] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 12. | Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, Liu Y, Gavrilova O, Patterson AD, Gonzalez FJ. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 367] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 13. | Han H, Hu C, Wang L, Zhang G, Liu S, Li F, Sun D, Hu S. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24:2152-2160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 15. | Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22:390-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Ueno T, Tanaka N, Imoto H, Maekawa M, Kohyama A, Watanabe K, Motoi F, Kamei T, Unno M, Naitoh T. Mechanism of Bile Acid Reabsorption in the Biliopancreatic Limb After Duodenal-Jejunal Bypass in Rats. Obes Surg. 2020;30:2528-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, Pigny P, Klein A, Kerr-Conte J, Gmyr V, Caiazzo R, Pattou F. Bile Diversion in Roux-en-Y Gastric Bypass Modulates Sodium-Dependent Glucose Intestinal Uptake. Cell Metab. 2016;23:547-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 704] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 20. | Hao Z, Leigh Townsend R, Mumphrey MB, Gettys TW, Yu S, Münzberg H, Morrison CD, Berthoud HR. Roux-en-Y Gastric Bypass Surgery-Induced Weight Loss and Metabolic Improvements Are Similar in TGR5-Deficient and Wildtype Mice. Obes Surg. 2018;28:3227-3236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Wang W, Cheng Z, Wang Y, Dai Y, Zhang X, Hu S. Role of Bile Acids in Bariatric Surgery. Front Physiol. 2019;10:374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 865] [Cited by in F6Publishing: 942] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 23. | Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, Mataki C, Sato H, Tanigawara Y, Schoonjans K, Itoh H, Auwerx J. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913-26920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, Bordenstein SR, Guo Y, Wasserman DH, Abumrad NN. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Goncalves D, Barataud A, De Vadder F, Vinera J, Zitoun C, Duchampt A, Mithieux G. Bile Routing Modification Reproduces Key Features of Gastric Bypass in Rat. Ann Surg. 2015;262:1006-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222-4230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 27. | Shonsey EM, Wheeler J, Johnson M, He D, Falany CN, Falany J, Barnes S. Synthesis of bile acid coenzyme a thioesters in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:360-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |