Copyright
©The Author(s) 2022.
World J Gastroenterol. Jul 14, 2022; 28(26): 3243-3257
Published online Jul 14, 2022. doi: 10.3748/wjg.v28.i26.3243
Published online Jul 14, 2022. doi: 10.3748/wjg.v28.i26.3243
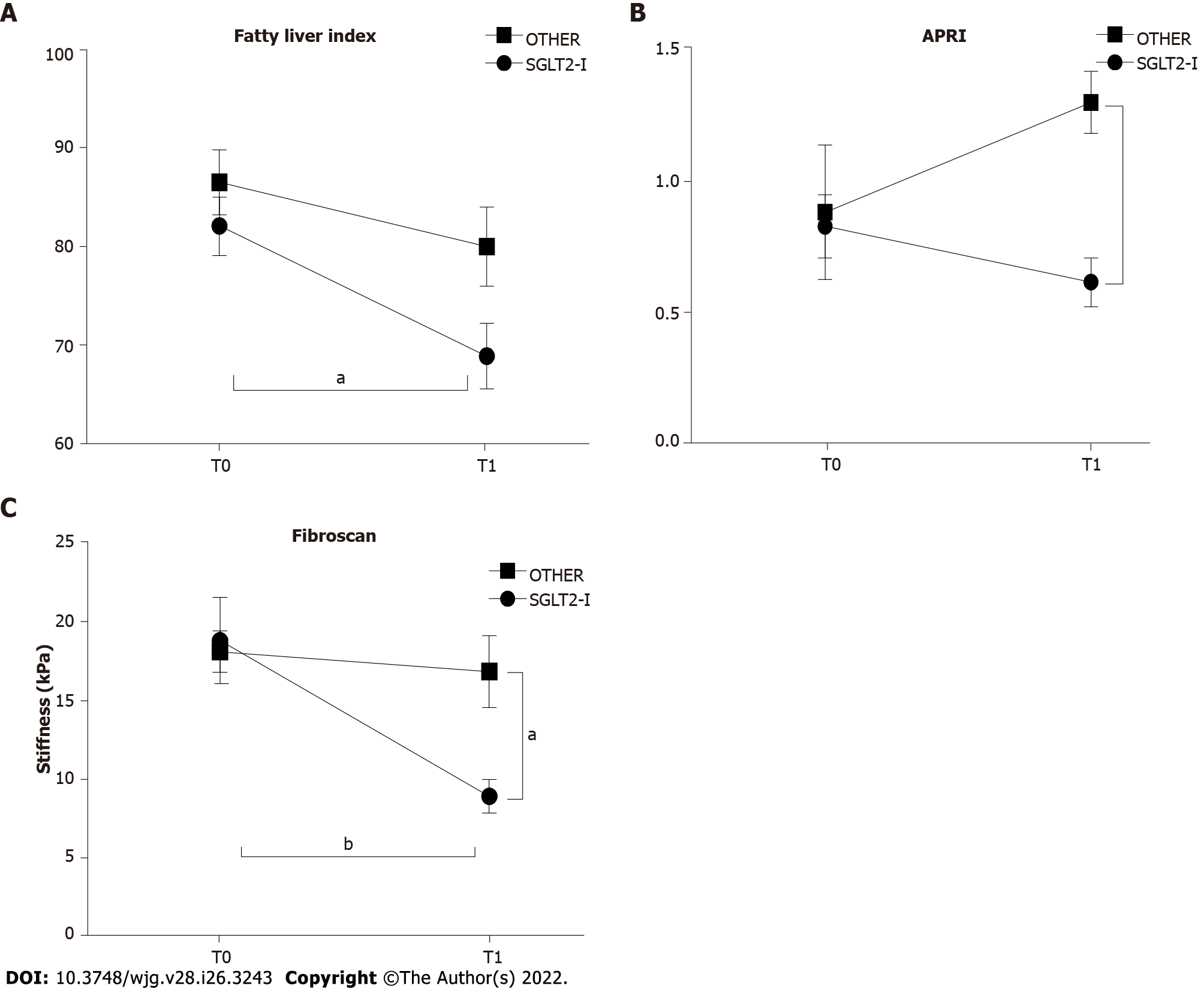
Figure 3 Non-invasive markers of liver steatosis and fibrosis in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose glucose-lowering before (T0) and after 1 wk of treatment (T1).
A: Fatty liver index; B: Aspartate aminotransferase-to-platelet ratio index; C: Hepatic elastometry in patients observed in the study grouped according to the assigned treatment. Data in the graphs are represented as mean ± SEM. Two-way analysis of variance and Tukey assessed statistical differences as posthoc test. aP < 0.05, bP < 0.01. SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug; APRI: Aspartate aminotransferase-to-platelet ratio index.
- Citation: Bellanti F, Lo Buglio A, Dobrakowski M, Kasperczyk A, Kasperczyk S, Aich P, Singh SP, Serviddio G, Vendemiale G. Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease. World J Gastroenterol 2022; 28(26): 3243-3257
- URL: https://www.wjgnet.com/1007-9327/full/v28/i26/3243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i26.3243