INTRODUCTION
Inflammatory bowel disease (IBD) refers to a group of chronic inflammatory diseases of the gastrointestinal tract, mainly including Crohn’s disease (CD) and ulcerative colitis (UC), which are lifelong diseases that tend to affect individuals aged 20–40 years[1,2]. IBD affects nearly all aspects of living, including patients’ private, occupational and social lives. In total, 50%–80% of UC patients and about 67% of CD patients suffer from relapses and remissions, which is a clinical feature of IBD. Around 15%–30% of UC patients fail to achieve sustained remission, while only 13% of CD patients have a relapse-free course[3,4]. Therefore, IBD has a large socioeconomic burden, and direct and indirect costs caused by UC reach 0.81–1.49 billion dollars per year in the United States[5].
Previous research on the mechanism of IBD suggests that genetic susceptibility, intestinal microbiota, environmental factors (such as diet and stress), and the association between proinflammatory and anti-inflammatory cells and factors are involved in the occurrence and development of IBD[6,7]. An increasing number of therapeutic agents have been investigated, including the tumor necrosis factor (TNF)-α antagonist infliximab, and the effectiveness of these agents has been confirmed[8]. Although the large number of treatment options available for IBD is a positive aspect, it complicates the choice of optimal treatment for individual patients.
TRADITIONAL THERAPEUTIC STRATEGIES
The primary therapeutic aim for IBD is to induce and maintain remission[9], of which mucosal healing is the most important target[10]. Besides clinical remission, the most important objective is to achieve mucosal healing under endoscopy[1,2,9,11], which is associated with reduction in recurrence, surgical interventions, intestinal damage and steroid dependence[12,13]. The current treatment regimens for UC and CD are stratified according to severity. A step-up strategy is usually used to treat mild-to-moderate IBD[1,2,14]. For example, mild-to-moderate UC would be first subjected to 5-aminosalicylic acid (5-ASA). When efficacy is unsatisfactory, treatments are escalated to glucocorticoid and/or immunosuppressants, while biologicals are usually used as the final resort. By contrast, for severe IBD or steroid-refractory IBD, immunosuppressants or biologicals may be considered in the earlier stage.
Although the majority of patients with mild-to-moderate UC who receive 5-ASA achieved symptomatic relief within 8 wk, 41% of patients who achieve induced remission eventually experience relapse[15,16]. Thus, daily medication over a longer period of time is necessary to maintain remission. In addition, multiple daily doses of 5-ASA are required in the stage of induced remission, which may impair compliance, particularly for outpatients without regular medication reminders. Additionally, when 5-ASA administration is reduced to once daily during the induction or maintenance of remission stage[16,17], patient compliance may drop to 40%[18]. Poor compliance is a key obstacle for the induction and maintenance of remission[19].
Glucocorticoids constitute an important treatment for the induction of remission in active disease, particularly in CD. However, about 16% of CD patients do not respond to glucocorticoids after treatment for 30 d[20]. In addition, glucocorticoid therapy is often accompanied by a variety of side effects, including diabetes, hypertension, opportunistic infections, and osteoporosis[21,22]. More than 28% of patients with CD develop steroid dependence[20]. Glucocorticoids are not effective in maintaining remission, and the risks associated with long-term use are marked; thus, the dose of glucocorticoids needs to be tapered at the onset of clinical remission[9,16]. When mucosal remission is not achieved, complications such as fistula and stenosis can easily occur[23,24].
Contrary to traditional step-up therapy, the TOP-DOWN trial[25] confirmed that early combination of immunosuppressants led to a higher remission rate than standard step-up therapy in patients without steroids. Evidence has confirmed that the early use of biologicals may benefit certain patients with mild-to-moderate IBD[26]. The CHARM[27], SONIC[24], PRECISE 2[28] and GEMINI 2[29] trials demonstrated the advantages of early biological treatment over step-up therapy in patients with CD, involving anti-TNFα (infliximab and certolizumab) and anti-integrins (vedolizumab). Without sufficient evidence in patients with UC, previous studies showed lower remission rates in patients who had a shorter course of the disease[30], while other studies suggested that early use of vedolizumab increased remission in patients with UC[31].
However, no consensus has formed regarding the early use of biologicals for patients with mild-to-moderate IBD. The Pharmaceutical Benefits Scheme (PBS) of Australia specifies that biologicals should be considered only when the disease remains clinically active after 6 wk of steroids and 3 mo of immunosuppressants[9]. Despite current evidence supporting early use of biologicals, particularly for patients with CD, clinical decision-making needs to involve patients when treatment is economically demanding. It would be improper for clinicians to use biologicals at an early stage solely relying on the personal opinion of the physician, as it could generate conflicts between physicians and patients.
Refractory IBD patients with severe complications (uncontrolled hemorrhage, perforation, abscesses or malignancy) are candidates for surgery[1,2]. However, a previous study showed that gastroenterologists might differ from IBD patients in the willingness to surgery. Patients were more willing than gastroenterologists to take risks to undergo surgery, and surgeons agreed with patients in the majority of cases[32]. The differences in perceptions among physicians and surgeons have a marked impact on patients, including the timing of referral for surgery.
Due to the convenience of wide access to information, patients may have their own ideas about treatment. Ignoring patients’ values or preferences may lead to patient dissatisfaction, poor compliance, and eventually reduced treatment efficacy. Therefore, it is advisable to involve patients in decision-making and to discuss patients’ preference after they have been fully informed about the treatment options, which can share the risks and improve patients’ compliance. This is called shared decision-making (SDM).
SDM
SDM is an approach where clinicians and patients share the best available evidence when faced with decision-making, and that patients are supported to consider options to achieve informed preferences[33]. Different from traditional diagnosis and treatment procedures, SDM requires that clinicians provide alternative choices of examinations and treatments, and describe the associated risks and benefits, while patients express their preferences and values, and both sides ultimately make decisions that are appropriate and are consistent with patients’ best interests[34].
SDM consists of three key steps: (1) Clinicians inform patients of alternative decisions and provide relevant, high-quality, accessible information; (2) Clinicians consider the patients’ values and preferences, particularly the most desired therapeutic targets; and (3) Physicians integrate the patients’ preferences and values into the decisions to be made[34-36]. The three-talk model is practical to distribute the aforementioned three steps into three conversations, including team talk, option talk and decision talk[37,38]. In the team talk, physicians first actively promote patient participation, inform patients of the current options available for consideration, help patients realize that this is a bidirectional treatment requiring their own participation, and allow patients to think about the primary therapeutic target. In the options talk, physicians introduce each choice to the patients in detail, including the associated advantages and disadvantages, so that patients can compare each option based on their values. For example, for patients with CD, it is necessary to discuss treatment options, such as medical, surgical, and endoscopic therapy, and evidence-based information should be used to explain the remission rate, risks, and costs of each option, so as to objectively demonstrate the advantages and disadvantages of each choice to allow the patients to balance the pros and cons of each approach according to their own preferences and values. In the decision talk, physicians should elicit the patients’ preferences and values; thus, the physicians need to understand what is most important to the patients, and make the appropriate decision together with the patients based on the patients’ preferences and values. It should be emphasized that it is a course of patients’ deliberation, from the stage of being aware of the options to that of understanding those options and having sufficient time to think about what is most important to them with the support of the physicians.
Decision aids can be used for patient education during SDM[39,40]. Decision aids are tools based on evidence-driven medical information. For example, patients can be intuitively told of the clinical remission rate of CD under infliximab monotherapy, and the approximate increased infection rate as well. These tools can be accessed online, on paper, or in video form[34,41,42], and aim to help patients to make deliberate choices among various treatment alternatives[43]. These tools can help patients to obtain relevant clinical evidence, include patients’ preferences when medical decision-making dilemmas occur, allow patients to understand the possible long- and short-term results, and promote high-quality decision-making. In terms of decision aids for biological agents, Almario et al[44] designed the online decision aid tool IBD and me, which covers the commonly used cetuzumab, viduzumab, adalimumab, infliximab, galimumab and eutecumab; and introduces the timing and frequency of biological use, route of administration, side effects (mainly infectious and oncogenic risks), and common adverse effects of hormones and immunosuppressants. A personalized decision preference report can be obtained after finishing the questionnaire online.
SDM IN IBD
Surveys have shown that the majority of IBD patients agree to be involved in decision-making, and want to be informed about alternative treatments[45-48]. The majority of patients who participate in SDM experience improved clinical satisfaction, higher trust in doctors, and better compliance[49-51]. However, SDM is not suitable for all cases. Medical decisions include effective decisions and preference-sensitive decisions[52]. For effective decisions, it has been demonstrated that the benefits outweigh the risks, so the decisions are undoubtedly the best strategy. For example, coloproctectomy is required in UC patients with complicated colorectal cancer. However, for preference-sensitive decisions, there is no sufficient evidence to demonstrate which treatment is the best, as there may be multiple reasonable treatment options (even including follow-up observation); thus, the judgement of the benefit/risk ratio falls upon patients, such as whether the treatment target they value has been achieved, or whether the side effects they are concerned about have occurred in the past. Of the two decision types, SDM only fits in the preference-sensitive decisions, which is often present in the management of IBD. IBD is a complex disease with large individual variation, and each treatment has particular advantages and disadvantages. Therefore, SDM is suitable for the management of IBD.
In addition to the aforementioned selection of step up therapy or top-down early intervention treatment strategy, SDM can be used for choosing biologicals. A number of biologicals are currently available for the treatment of IBD, including infliximab, adalimumab, certolizumab, golimumab, natalizumab, vedolizumab and ustekinumab, with differences in the mechanism, route of administration, and side effects[53]. However, it was not until 2019 that the first head-to-head VARSITY trial[54] compared the efficacy and safety of intravenous vedolizumab to subcutaneous adalimumab. Other head-to-head trials of different biologicals are still awaited[55]. Clinicians’ selection of biologicals especially for CD patients is varied, because the majority of real-world data on CD show no significant differences in clinical remission rate[56]. Under these circumstances, it is a preference-sensitive decision to choose biologicals. For example, infliximab is administered intravenously, while adalimumab is administered subcutaneously, thus the length of hospital stay varies. While infliximab is administered at intervals of 0, 2 and 6 wk to induce remission, and then administered every 8 wk, adalimumab is administered every 2 wk. In such a case, some patients may be more concerned with the frequency or route of administration due to the distance to the hospital, and therefore may choose infliximab treatment at longer intervals, whereas other patients may give priority to side effects. In a study involving 640 patients with IBD[44], factors that influenced the choice of biologicals for patients with UC were in the following order: long-term remission rate, route/frequency of administration, and risk of lymphoma. For patients with CD, these factors were short-term remission rate, risk of lymphoma, and route/frequency of administration. Of note, a small percentage of patients (3.6%) cited the mechanism of action as the primary factor for their selection of a given biological. At the same time, physicians’ assumption of patients’ acceptance of biologicals and their preferred route of administration differs from the patients’ opinion[57]. If doctors impose on their patients what they think is universally applicable instead of incorporating the patients’ preference into the decision-making process, patients may be forced to agree to a treatment plan against their own values. Patients who do not trust their doctors may develop a self-protective mechanism of rejection, thus leading to poor compliance. This awkward situation can be avoided to a large extent by involving patients in the decision-making process.
For the past 20 years, it has been discussed whether biologicals alone or in combination with immunosuppressants should be used for the treatment of IBD. The results of the SONIC study showed the advantages of the combination treatment[58]. Infliximab combined with immunosuppressants achieved higher rates of clinical remission and mucosal healing compared with infliximab or azathioprine alone in corticosteroid-free patients with CD. This may be associated with the fact that immunosuppressants reduce the generation of antidrug antibodies and increase the blood concentration of biological agents[58,59]. Although there is increasing evidence that combination treatment is superior to single-drug treatment[59-61], and the early use of infliximab with azathioprine or methotrexate for ≥ 1 year has been recommended[62], to date, not all the studies support the use of combination treatment, particularly in the case of biologicals other than infliximab[63-67]. In terms of safety, previous studies have shown that biologicals combined with immunosuppressants can increase the incidence of infection and malignancy[68-72]. Therefore, the benefit/risk ratio remains unclear regarding the combination of biological agents with immunosuppressants; thus, clinicians need to explain the benefits and risks to patients. More importantly, the preferences of the patients need to be considered to determine whether the patients would be willing to accept the risk of increased infection and tumor incidence to achieve an increase in remission rate. By involving patients in the decision-making process, the risk of increased side effects can be shared by both doctors and patients.
IBD patients have a risk of relapse after reduction or withdrawal of 5-ASA, glucocorticoids, immunosuppressants, biologicals, or combination therapy. Approximately 30% of patients with CD or UC who stop immunosuppressant monotherapy relapse within 2 years, while 50%–75% relapse within 5 years. By contrast, the risk of relapse after stopping anti-TNF therapy is 30%–40% at 1 year and > 50% at 2 years[73]. The therapeutic cost associated with the long-term use of aforementioned drugs is high, and some side effects may be related to the duration of use, such as the risk of cancer after the use of biologicals. Therefore, whether the treatment should be stopped or the dose tapered needs to be weighed with regard to the possible benefits, costs and risks, and decisions must be made individually. Given the high recurrence rate of IBD and the uncertainty about the optimal timing for clinical, biochemical and endoscopic follow-up, follow-up plans after drug withdrawal or dose reduction should also be made together with patients, taking their preferences into account[73].
As mentioned above, in addition to requiring informed consent, whether patients with IBD should undergo surgery also requires SDM. Controversies exist regarding the choice of surgery[74]. Ileal pouch–anal anastomosis (IPAA) has become the standard surgical option offered to patients with UC, with endoanal mucosal resection being the second choice. IPAA preserves the anal transition zone, which improves fecal continence. However, the elimination of colonic mucosa is the goal of surgical intervention; thus, what IPAA preserves will put the patient at risk of developing dysplasia and residual disease in the remaining anal canal epithelium, while mucosectomy can decrease dysplasia risk. Therefore, doctors and patients should balance the benefits against potential relapse and malignant change in the retained mucosa.
DISCUSSION
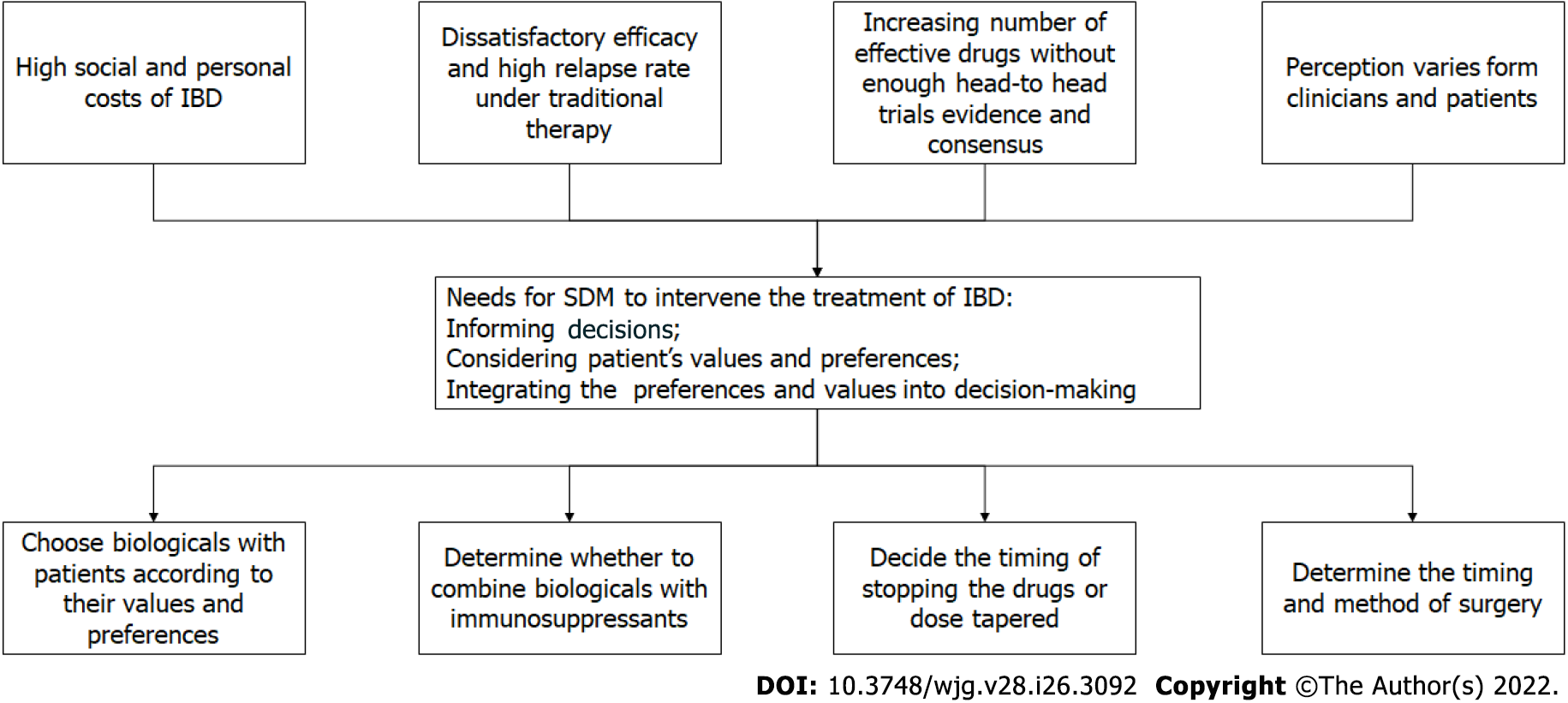
In multiple aspects of IBD management, SDM has shown potential. We believe that SDM can and should be used in IBD therapy (Figure 1). As mentioned, the treatment of IBD is a preference-sensitive choice; the advantages and disadvantages of which need to be weighed by the patients. Clinicians need to invite patients to the decision-making process, even though not all situations are suitable for SDM. For example, when perforation occurs in a UC patient with toxic megacolon, there is no doubt about the choice of treatment, but patients still need to be aware of the need to undergo surgery. Therefore, clinicians must determine whether it is a preference-sensitive decision and whether SDM is required. If patients decline to participate in the SDM even after multiple invitations by doctors, clinicians may not have to continue spending their time on persuading patients.
Figure 1 The reasons and fields of application for shared decision making in inflammatory bowel disease treatments.
IBD: Inflammatory bowel disease; SDM: Shared decision making.
SDM is a type of informed consent based on evidence-based medicine, putting forward certain requirements for doctors and patients. Challenges inevitably exist in the implementation of SDM. From the aspect of clinicians, as mentioned above, it is important to consider whether doctors are fully aware of the pros and cons of the drugs they choose as new treatments emerge and research advances rapidly. Although it can be resolved with the help of decision aids tools, the tools also should be updated continually. In addition, clinicians should have the awareness and techniques of SDM, and need to receive professional education. With the SDM skills, physicians’ willingness to impart relevant knowledge to patients should be emphasized. In addition, different perceptions of IBD between physicians and surgeons may influence patients’ perceptions. However, it is worth mentioning that diagnosis and treatment after discussion with a multidisciplinary team is a model that will help bridge the cognitive gap between physicians and surgeons. IBD patients’ preferences and disease patterns vary[44], meaning that no SDM plan fits all patients once and for all. Doctors need to be flexible, and patients need to have unlimited access to information and they should be actively invited to the decision-making process. The implementation of SDM undoubtedly needs policy support. For example, biologicals obtained in a first-tier city may not be available in other less-developed cities, which is a restriction for SDM. Unfortunately, SDM is not adequately addressed in current medical education, leading to less awareness of what it is and how to implement it. Therefore, we can’t emphasize it too much that the healthcare providers should be trained in their early stage of career to increase the benefits of implementing of SDM. And the early stage training hopefully will cultivate physicians’ habit unconsciously to take advantage of SDM in the medical practice.
These challenges need to be carefully considered before SDM can be implemented. Although SDM is theoretically suitable for patients with IBD, studies comparing SDM with traditional decision-making methods remain sparse and are eagerly awaited in the future. We hope that this review will promote the use of SDM in the management of IBD.
CONCLUSION
The investigations delineated in the present article revealed the dilemma of choosing individualized treatments for patients with IBD. We also discussed the advantages of SDM and the aspects in which SDM can be used. Current evidence showed the limitations of conventional step-up therapy for IBD. However, lack of head-to-head clinical trials and diverse treatment preferences of patients lead to difficulty of individualized decision-making. We demonstrate the latest advance of SDM with the support of clinical data, hoping that SDM will be better used by physicians caring for IBD patients.
ACKNOWLEDGEMENTS
We thank Pro. Ji Li for his comment upon this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaur M, United States; Razzaque MS, United States S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ