Copyright
©The Author(s) 2022.
World J Gastroenterol. Jun 28, 2022; 28(24): 2705-2732
Published online Jun 28, 2022. doi: 10.3748/wjg.v28.i24.2705
Published online Jun 28, 2022. doi: 10.3748/wjg.v28.i24.2705
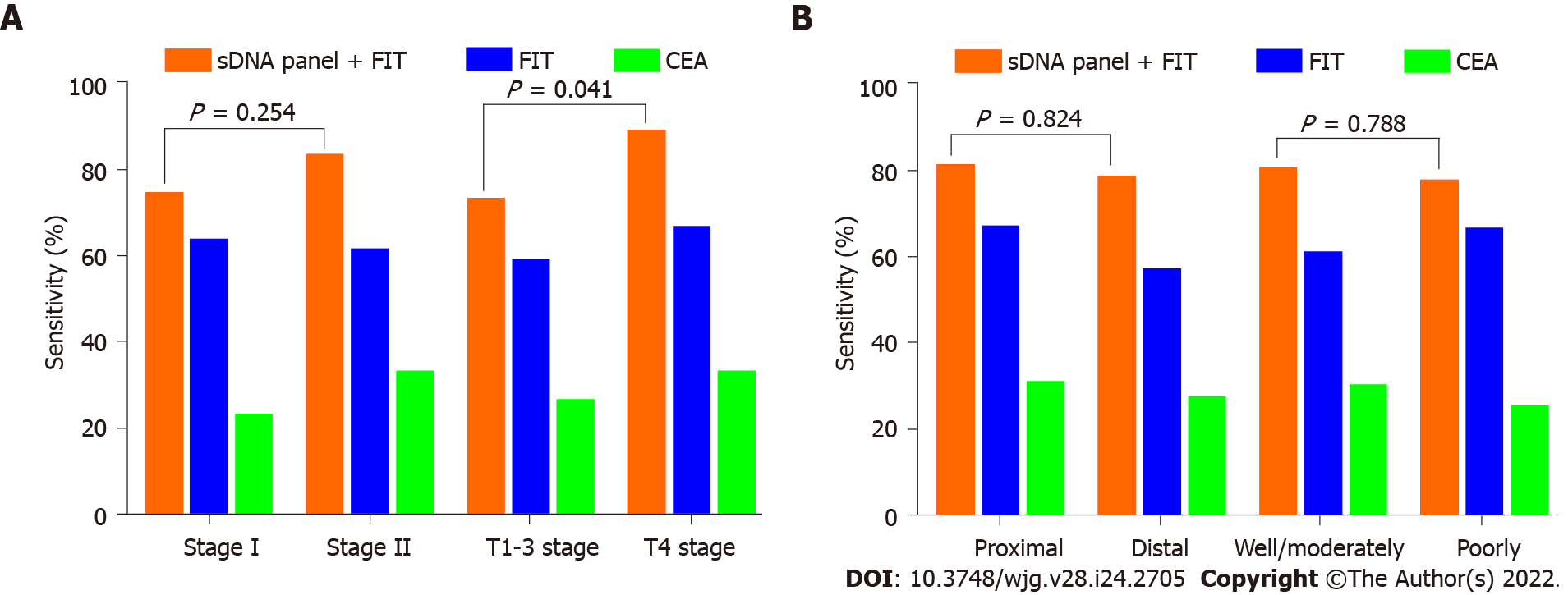
Figure 5 Impact of clinicopathologic covariates on screening.
A: Sensitivities of fecal immunochemical test (FIT), serum carcinoembryonic antigen (CEA) and stool DNA (sDNA) panel + FIT for the detection of early-stage colon cancer (ECC), according to tumor-node-metastasis stage or T stage; B: Sensitivities of FIT, serum CEA and sDNA panel + FIT for the detection of ECC, according to tumor site or histological differentiation. sDNA: Stool DNA; FIT: Fecal immunochemical test; CEA: Carcinoembryonic antigen.
- Citation: Jiang HH, Xing SW, Tang X, Chen Y, Lin K, He LW, Lin MB, Tang EJ. Novel multiplex stool-based assay for the detection of early-stage colon cancer in a Chinese population. World J Gastroenterol 2022; 28(24): 2705-2732
- URL: https://www.wjgnet.com/1007-9327/full/v28/i24/2705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i24.2705