Copyright
©The Author(s) 2022.
World J Gastroenterol. Jun 21, 2022; 28(23): 2582-2596
Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2582
Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2582
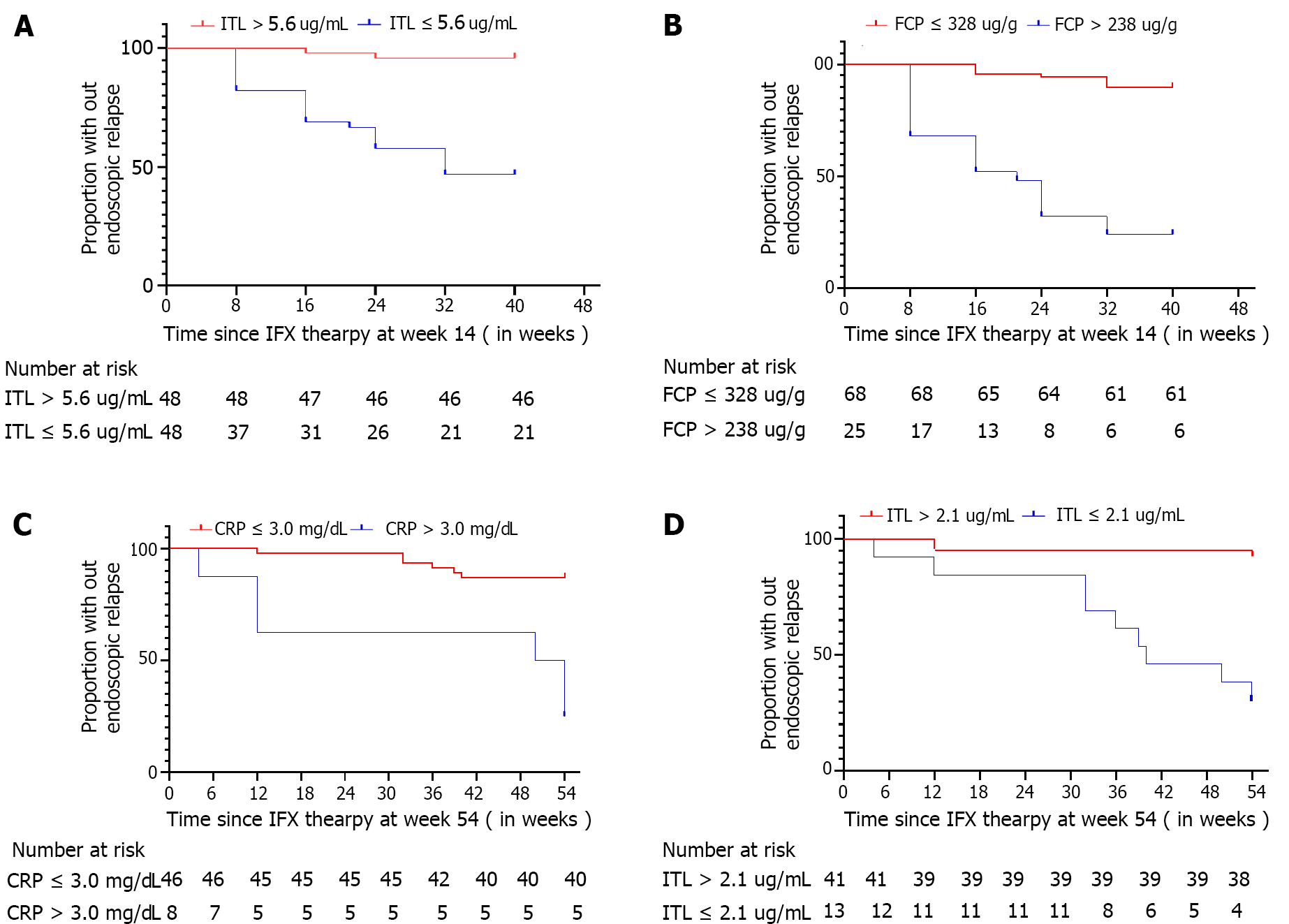
Figure 4 Proportion without endoscopic relapse.
A. Time since infliximab (IFX) therapy at week 14 [IFX trough level (ITL) > 5.6 μg/mL vs ITL ≤ 5.6 μg/mL]; B: Time to IFX therapy at week 14 (fecal calprotectin ≤ 238 μg/g vs fecal calprotectin > 238 μg/g]; C: Time to IFX therapy at week 54 (C-reactive protein ≤ 3.0mg/L vs C-reactive protein > 3.0 mg/L); D: Time to IFX therapy at week 54 (ITL > 2.1 μg/mL vs ITL ≤ 2.1 μg/mL). ITL: Infliximab trough level; CRP: C-reactive protein; FCP: Fecal calprotectin; IFX: Infliximab.
- Citation: Cao WT, Huang R, Liu S, Fan YH, Xu MS, Xu Y, Ni H. Infliximab trough level combined with inflammatory biomarkers predict long-term endoscopic outcomes in Crohn’s disease under infliximab therapy. World J Gastroenterol 2022; 28(23): 2582-2596
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2582