Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2597
Peer-review started: September 14, 2021
First decision: November 7, 2021
Revised: November 21, 2021
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: June 21, 2022
Tumor necrosis factor-alpha inhibitors, including infliximab and adalimumab, are effective medical treatments for perianal fistulising Crohn’s disease (CD), but not all patients achieve fistula healing.
To determine the correlation between perianal fistula healing and closure with infliximab and adalimumab trough levels.
In this multicentre retrospective study conducted across four tertiary inflammatory bowel disease centres in Australia, we identified CD patients with perianal fistulae on maintenance infliximab or adalimumab who had a trough level within twelve weeks of clinical assessment. Data collected included demographics, serum infliximab and adalimumab trough levels (mg/L) within 12 wk before or after their most recent clinical assessment and concomitant medical or surgical therapy. The primary outcome was fistula healing, defined as cessation in fistula drainage. The secondary outcome was fistula closure, defined as healing and closure of all external fistula openings. Differences between patients who did or did not achieve fistula healing were compared using the chi-square test, t test or Mann-Whitney U test.
One hundred and fourteen patients (66 infliximab, 48 adalimumab) were included. Forty-eight (72.7%) patients on maintenance infliximab achieved fistula healing and 18 (27.3%) achieved fistula closure. Thirty-seven (77%) patients on maintenance adalimumab achieved fistula healing and 17 (35.4%) achieved fistula closure. Patients who achieved fistula healing had significantly higher infliximab and adalimumab trough levels than patients who did not [infliximab: 6.4 (3.8-9.5) vs 3.0 (0.3-6.2) mg/L, P = 0.003; adalimumab: 9.2 (6.5-12.0) vs 5.4 (2.5-8.3) mg/L, P = 0.004]. For patients on infliximab, fistula healing was associated with lower rates of detectable anti-infliximab antibodies and younger age. For patients on adalimumab, fistula healing was associated with higher rates of combination therapy with an immunomodulator. Serum trough levels for patients with and without fistula closure were not significantly different for infliximab [6.9 (4.3-10.2) vs 5.5 (2.5-8.3) mg/L, P = 0.105] or adalimumab [10.0 (6.6-12.0) vs 7.8 (4.2-10.0) mg/L, P = 0.083].
Higher maintenance infliximab and adalimumab trough levels are associated with perianal fistula healing in CD.
Core Tip: This multicentre retrospective study demonstrated a significant association between both infliximab and adalimumab trough levels with fistula healing, with higher levels associated with increased healing rates. Higher tertiles of both infliximab and adalimumab levels were associated with a higher proportion of patients achieving fistula healing. Fistula healing, defined as cessation of fistula drainage, is a clinically relevant endpoint that impacts on patient quality of life. Our results support dose-escalation of both infliximab and adalimumab in non-responders, targeting higher levels to achieve fistula healing prior to changing biologic therapy. Importantly, this study is the largest study to date assessing the relationship between adalimumab trough levels and clinical fistula healing.
- Citation: Gu B, Venkatesh K, Williams AJ, Ng W, Corte C, Gholamrezaei A, Ghaly S, Xuan W, Paramsothy S, Connor S. Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn’s disease. World J Gastroenterol 2022; 28(23): 2597-2608
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2597
Perianal fistulising disease is a common manifestation occurring in up to 30% of patients with Crohn’s disease (CD). The development of abnormal tracts between the bowel and perineum can cause perianal drainage, pain, bleeding, abscess formation, sepsis and faecal incontinence[1,2]. Perianal CD is associated with significant morbidity and decreased quality of life, negatively impacting physical, emotional, sexual and social wellbeing[1-3] and is an independent predictor for decreased productivity in patients with CD[4,5]. Given that the incidence of perianal fistulising CD is highest in the third and fourth decades of life, this places significant burden on patients, society, the economy and the health care system[6].
Treatment for perianal fistulising CD requires a multidisciplinary approach involving medical management with immunosuppressants and antibiotics, as well as surgical management with sepsis control, seton insertion and sometimes diversion or resection. Anti-tumor necrosis factor (anti-TNF) alpha agents, including infliximab[7,8] and adalimumab[9,10], are the most effective medical therapies available for inducing and maintaining remission of fistulas. Unfortunately, up to 60% of patients treated with maintenance infliximab lose response within one year[7,8]. Accumulating evidence suggests that this loss of response is partly due to subtherapeutic anti-TNF trough levels. Retrospective studies and post-hoc analyses of prospective data have identified that higher infliximab trough levels are associated with fistula healing and closure compared to what is observed for mucosal healing in luminal disease, with emerging data suggesting similar results for adalimumab[11-14]. Quantitative assays for therapeutic drug monitoring (TDM) permit individualisation of infliximab and adalimumab dosing[15,16], however there are very few studies on perianal fistulising CD and the optimal target levels for perianal fistulising CD remain unclear. Our study aims to assess the association between serum trough infliximab and adalimumab levels and perianal fistula healing and closure and identify optimal target levels.
This was a multicentre retrospective cross-sectional study of patients with perianal fistulising CD at four tertiary inflammatory bowel disease centres across Australia between January 2014 and June 2020. All patients qualified for infliximab or adalimumab under the Australian Pharmaceutical Benefits Scheme criteria[17] which constitutes the following: (1) A confirmed diagnosis of CD using clinical, radiological, histological and/or endoscopic criteria; and (2) At least one active externally draining complex perianal fistula. We included patients on maintenance infliximab or adalimumab with a documented perianal examination who had a serum infliximab or adalimumab trough level collected within 12 wk before or after their most recent clinical assessment. Infliximab and adalimumab trough levels as well as antibodies to infliximab and adalimumab were measured using a drug sensitive enzyme-linked immunosorbent assay (Grifols Promonitor for adalimumab; LISA-Tracker and Grifols Promonitor for infliximab). Infliximab and adalimumab trough levels were measured both in a proactive manner and reactive manner in patients failing treatment across the study sites. Patients who had been changed from infliximab to adalimumab or vice versa and had relevant data were included in both the infliximab and adalimumab groups.
All patients had received standard infliximab or adalimumab induction dosing (infliximab 5 mg/kg intravenously at weeks 0, 2, and 6; adalimumab subcutaneously 160 mg at week 0, 80 mg at week 2) followed by maintenance therapy. The current dose of anti-TNF therapy was recorded and patients with or without dose-escalated maintenance therapy were included. Patients who had a diversion ostomy, rectovaginal fistula or no documented perianal examination were excluded.
Data was retrospectively collected from a clinical database that was updated prospectively during routine clinical practice. Patient demographics collected included age, gender, weight, body mass index, smoking status and CD phenotype classified according to the Montreal Classification[18]. The location of CD was identified as ileal, ileocolonic, colonic, upper gastrointestinal involvement or no luminal disease. The presence or absence of fistulising and stricturing disease was noted, in particular the presence of anal strictures. Biochemical markers of disease activity including C-reactive protein (CRP) and albumin were also recorded.
Prior history of surgical management of perianal disease or fistula was recorded and categorised as examination under anaesthesia and curettage, examination under anaesthesia and seton insertion or fistulotomy. The duration from the last surgical procedure to the follow up visit was recorded. Concomitant medical therapy at the time of follow up was assessed, including corticosteroid use, 5-aminosalicylates and immunomodulators. The doses of infliximab and adalimumab were recorded and stratified according to dose and interval between doses. For patients on dose-escalated anti-TNF therapy, the duration between last dose escalation and follow up was recorded.
The primary outcome was fistula healing, which was defined as cessation of fistula drainage, with or without a seton in situ[7]. The secondary outcome was fistula closure, which was defined as healing and closure of all external fistula openings[7].
Statistical review of this study was performed by a biostatistician from the Ingham Institute for Applied Medical Research. Descriptive statistics were used to assess the baseline characteristics of both the infliximab and adalimumab cohorts. Categorical variables were expressed as percentages and compared using the chi-square test. Continuous variables were expressed using mean ± SD for normally distributed variables and median and interquartile range (IQR) for non-normally distributed variables. The means were compared using the t test for normally distributed variables and the mean ranks compared using the Mann-Whitney U test for non-normally distributed variables. A receiver operating characteristic (ROC) curve analysis was used to assess the sensitivity and specificity of infliximab and adalimumab levels at different cut-off points for predicting fistula healing. All reported P values were 2-sided, with P < 0.05 considered statistically significant. Multivariate analysis using logistic regression with forwards selection was used to analyse variables that predicted fistula healing. Variables which were statistically significant in the univariate analysis were included in the multivariate analysis model. Ethics approval was obtained from the South Western Sydney Local Health District (Human Research Ethics Committee LNR/18/LPOOL/404; Local Project Number: HE18/261).
Out of 454 patients screened, 114 patients (66 infliximab, 48 adalimumab) on maintenance infliximab or adalimumab for perianal CD had a trough level collected within 12 wk of clinical assessment. Five patients had been changed from infliximab to adalimumab or vice versa and were included in both the infliximab and adalimumab groups. Seventy-five (66%) patients were on combination therapy (43 azathioprine, 16 6-mercaptopurine, 16 methotrexate). Nineteen patients (28.8%) on maintenance infliximab were on dose escalated infliximab therapy (5, 7.5, 10, 15 or 20 mg/kg every 6 or 8 wk). For these patients, the median duration between last infliximab dose adjustment and follow up was 60.0 wk (IQR = 44.5-81.0). Eleven (22.9%) patients on maintenance adalimumab were on dose escalated adalimumab therapy (40 mg weekly). For these patients, the median duration between last adalimumab dose adjustment and follow up was 39.0 wk (IQR = 24.0-86.0). Fifty-nine (89.3%) patients on infliximab had prior surgical management of their fistula, with a median duration of 93.0 wk (IQR = 45.5-284.5) between their last surgical procedure and their most recent follow up visit. Thirty-seven (77.1%) patients on adalimumab had prior surgical management of their fistula, with a median duration of 83.0 wk (IQR = 28.75-223.0) between their last surgical procedure and their most recent follow up visit. Patient demographics and disease characteristics of the population are summarised in Table 1.
| Infliximab (n = 66) | Adalimumab (n = 48) | |
| Median age, yr (IQR) | 36.0 (28.8-43.3) | 34.5 (29.0-51.8) |
| A1, n (%) | 2 (3.0) | 7 (14.6) |
| A2, n (%) | 52 (78.8) | 26 (54.2) |
| A3, n (%) | 9 (13.6) | 13 (27.1) |
| Female gender, n (%) | 28 (42.4) | 14 (29.2) |
| Mean weight, kg (SD) | 80.9 (18.7) | 82.1 (21.4) |
| Mean BMI, kg/m2 (SD) | 28.1 (4.8) | 28.5 (5.5) |
| Median age at diagnosis of Crohn’s disease (IQR) | 26.0 (21.0-34.0) | 24.0 (19.0-41.3) |
| Current smoker, n (%) | 12 (18.2) | 5 (10.4) |
| Disease location | ||
| Ileal, n (%) | 16 (24.2) | 17 (35.4) |
| Colonic, n (%) | 26 (39.4) | 7 (14.6) |
| Ileocolonic, n (%) | 15 (22.7) | 18 (37.5) |
| No luminal disease, n (%) | 4 (6.1) | 2 (4.2) |
| Upper gastrointestinal involvement, n (%) | 4 (6.1) | 2 (4.2) |
| Stricturing, n (%) | 10 (15.2) | 11 (22.9) |
| Penetrating, n (%) | 7 (10.6) | 17 (35.4) |
| Median duration on anti-TNF agent, wk (IQR) | 144.0 (80.0-280.0) | 180.0 (107.3-309.8) |
| Anti-TNF dosing, n (%) | ||
| IFX, 5 mg/kg/8 wk | 47 | - |
| IFX, 7.5 mg/kg/8 wk | 1 | - |
| IFX, 10 mg/kg/8 wk | 12 | - |
| IFX, 15 mg/kg/8 wk | 1 | - |
| IFX, 20 mg/kg/8 wk | 1 | - |
| IFX, 5 mg/kg/6 wk | 3 | - |
| IFX, 10 mg/kg/6 wk | 1 | - |
| ADA, 40 mg fortnightly | - | 37 |
| ADA, 40 mg weekly | - | 11 |
| Concurrent steroids, n (%) | 0 (0.0) | 1 (2.1) |
| Concurrent aminosalicylates, n (%) | 4 (6.1) | 5 (10.4) |
| Combination with immunomodulator, n (%) | 46 (69.7) | 29 (60.4) |
| Methotrexate, n (%) | 8 (12.1) | 8 (16.7) |
| 6-mercaptopurine, n (%) | 9 (13.6) | 7 (14.6) |
| Azathioprine, n (%) | 29 (43.9) | 14 (29.2) |
| Concurrent allopurinol, n (%) | 10 (15.2) | 4 (8.3) |
| Mean albumin, g/L (SD) | 39.9 (4.9) | 40.2 (4.7) |
| Median CRP, mg/L (IQR) | 1.4 (0.7-5.5) | 2.3 (1.2-5.2) |
Forty-eight (72.7%) patients on maintenance infliximab achieved fistula healing. Table 2 summarises the differences between patients on infliximab with and without fistula healing. Patients who achieved fistula healing had higher infliximab trough levels [6.4 (3.8-9.5) vs 3.0 (0.3-6.2) mg/L, P = 0.003], lower rates of detectable anti-infliximab antibodies (4.3% vs 33.3%, P = 0.004) and a younger age (33.0 vs 43.5 years old; P = 0.003) compared to patients who did not achieve fistula healing. The presence of detectable anti-infliximab antibodies was associated with lower infliximab trough levels (P = 0.02). The CRP and albumin levels were not significantly different between patients with and without fistula healing. The rates of combination therapy with an immunomodulator were not significantly different between patients who achieved fistula healing and those who did not (P = 0.522).
| Patients with fistula healing (n = 48) | Patients without fistula healing (n = 18) | P value | |
| Median age, yr (IQR) | 33.0 (28.0-38.0) | 43.5 (34.3-57.3) | 0.005 |
| Female gender, n (%) | 20 (41.7) | 8 (44.4) | 0.839 |
| Mean weight, kg (SD) | 82.2 (19.4) | 76.5 (15.7) | 0.378 |
| Mean BMI, kg/m2 (SD) | 28.5 (5.0) | 26.7 (3.8) | 0.318 |
| Median age at diagnosis of Crohn’s disease (IQR) | 26.0 (20.75-30.5) | 30.0 (24.0-43.0) | 0.121 |
| A1, n (%) | 1 (2.1) | 1 (5.6) | - |
| A2, n (%) | 41 (85.4) | 11 (61.1) | - |
| A3, n (%) | 4 (8.3) | 5 (27.8) | - |
| Current smoker, n (%) | 8 (16.7) | 4 (22.2) | 0.696 |
| Location | |||
| Ileal, n (%) | 14 (29.2) | 2 (11.1) | - |
| Colonic, n (%) | 19 (39.6) | 7 (38.9) | - |
| Ileocolonic, n (%) | 11 (22.9) | 4 (22.2) | - |
| No luminal disease, n (%) | 2 (4.2) | 2 (11.1) | - |
| Upper gastrointestinal involvement, n (%) | 3 (6.3) | 1 (5.6) | - |
| Stricturing, n (%) | 7 (14.6) | 3 (16.7) | 0.822 |
| Penetrating, n (%) | 5 (10.4) | 2 (11.1) | 0.927 |
| Median duration on anti-TNF agent, wk (IQR) | 153.0 (86.0-285.0) | 95.5 (40.25-322.75) | 0.387 |
| Dose escalated anti-TNF therapy, n (%) | 15 (31.3) | 4 (22.2) | - |
| Concurrent steroids, n (%) | 0 (0.0) | 1 (5.6) | - |
| Concurrent aminosalicylates, n (%) | 4 (8.3) | 1 (5.6) | 0.219 |
| Combination with immunomodulator, n (%) | 35 (72.9) | 11 (61.1) | 0.522 |
| Methotrexate, n (%) | 6 (12.5) | 2 (11.1) | 0.937 |
| 6-mercaptopurine, n (%) | 6 (12.5) | 3 (16.7) | 0.597 |
| Azathioprine, n (%) | 23 (47.9) | 6 (33.3) | 0.368 |
| Concurrent allopurinol, n (%) | 9 (18.8) | 1 (5.6) | 0.197 |
| Mean albumin, g/L (SD) | 40.3 (4.7) | 40.7 (4.6) | 0.590 |
| Median CRP, mg/L (IQR) | 2.1 (1.0-4.5) | 5.5 (1.1-8.7) | 0.094 |
| Median trough level, mg/L (IQR) | 6.4 (3.8-9.5) | 3.0 (0.3-6.2) | 0.003 |
| Detectable antibody, n (%) | 3 (4.3) | 6 (33.3) | 0.004 |
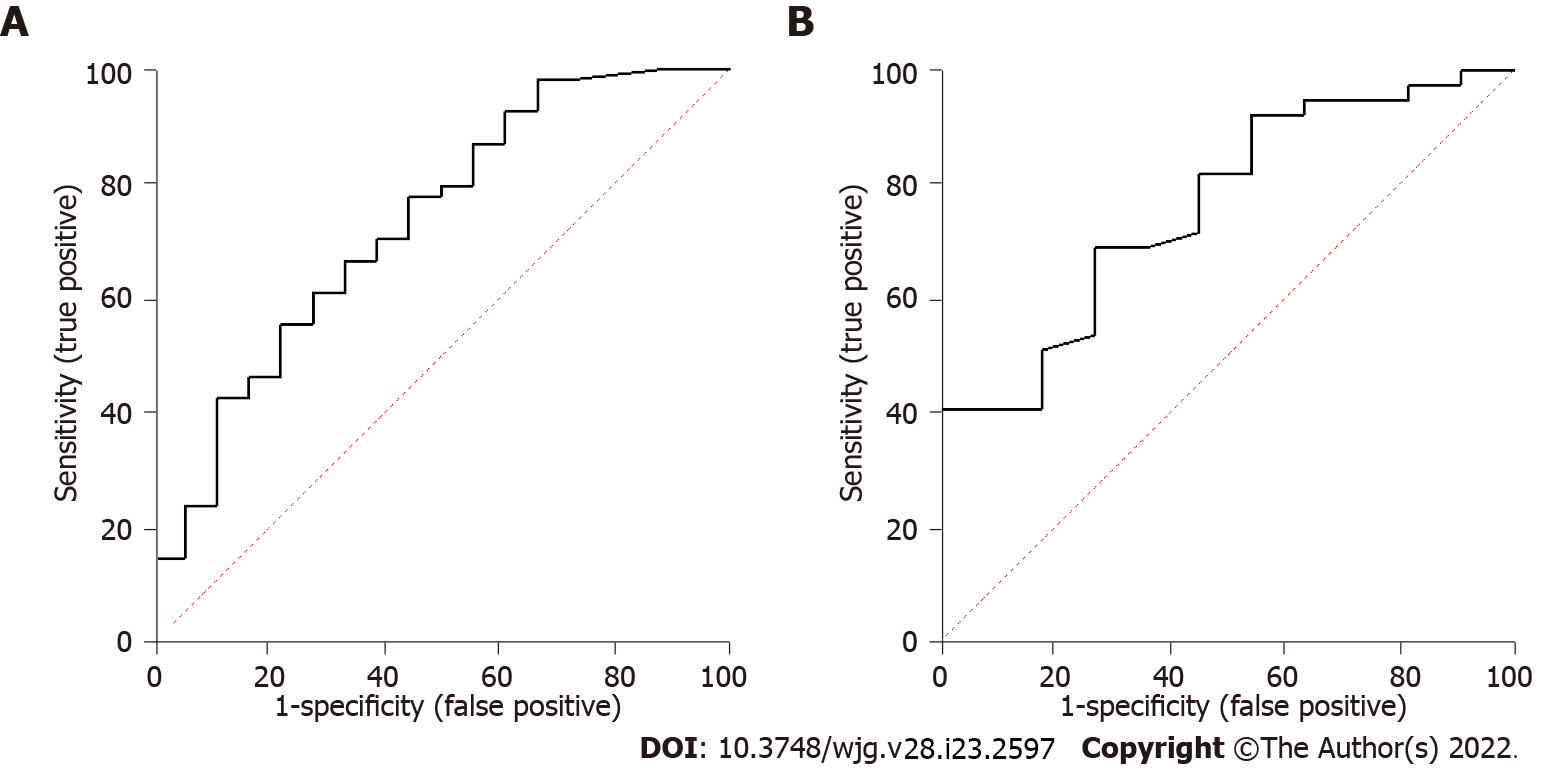
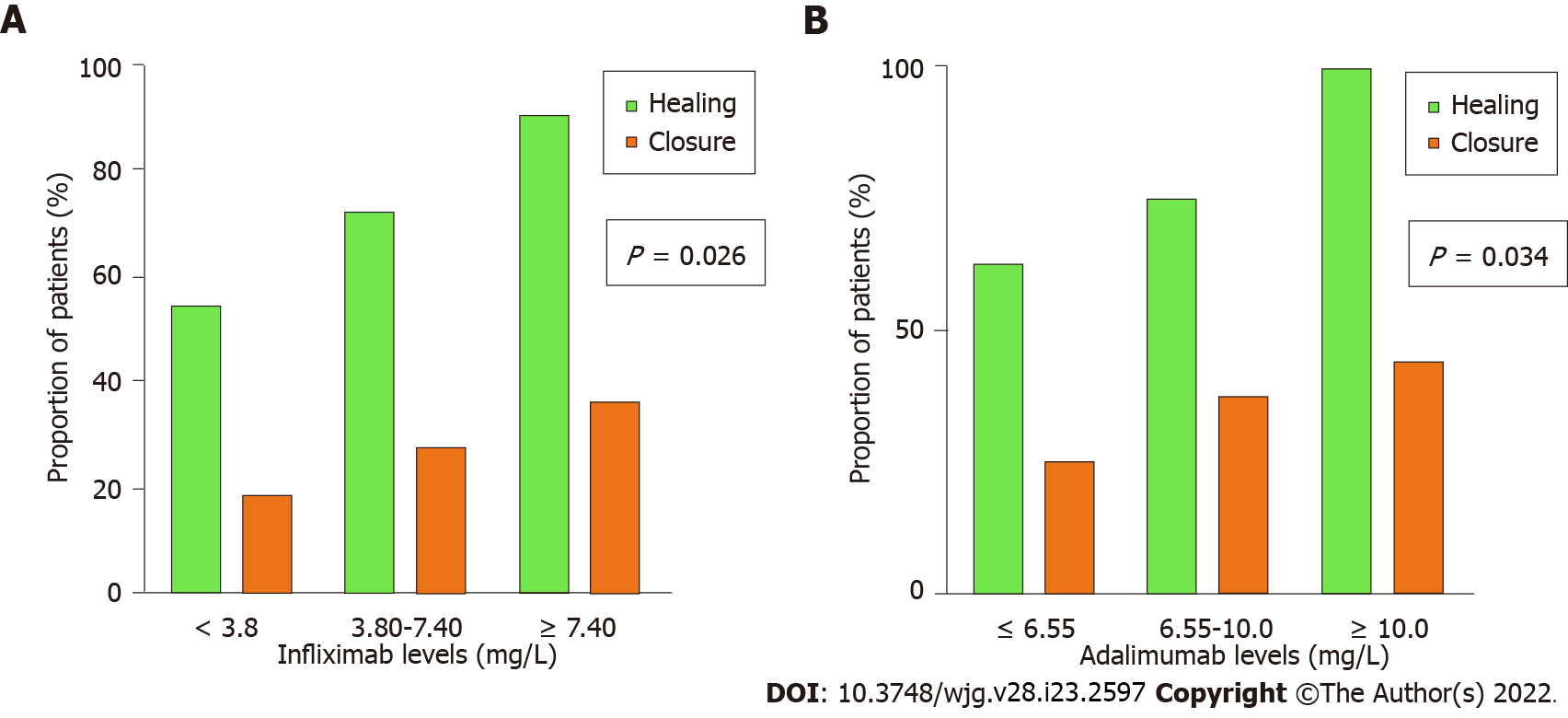
ROC curve analysis identified a positive correlation between infliximab trough levels and healing [area under the curve (AUC) = 0.74, 95% confidence interval (CI): 0.60-0.88, P = 0.003; Figure 1A] with an infliximab trough level of 6.10 mg/L that maximised the sensitivity and specificity of predicting fistula healing [sensitivity 58%, specificity 78%, odds ratio (OR) = 4.9, P = 0.013]. Upon tertile analysis, higher tertiles of infliximab levels were associated with a higher proportion of patients achieving fistula healing with 54.5% healing rate for tertile 1 compared to 90.1% for tertile 3 (Figure 2A; P = 0.026). Out of the patients who achieved fistula healing on infliximab, 90% and 95% of the patients who achieved fistula healing were healed with an infliximab trough level of 12.7 and 14.4 mg/L respectively. Given that a drug-sensitive infliximab assay was used where anti-infliximab antibody titres were only performed if infliximab concentrations were < 2.0 mg/L, anti-infliximab antibodies were not included in the multivariate analysis. On multivariate logistic regression analysis, age was associated with healing (P = 0.026) but adequate infliximab levels ≥ 6.10 mg/L were not (P = 0.097). Within our cohort, 18 (27.3%) of patients on infliximab achieved fistula closure. The infliximab trough level for patients with and without fistula closure was not significantly different [6.9 (4.3-10.2) vs 5.5 (2.5-8.3) mg/L, P = 0.105].
Thirty-seven (77%) patients on maintenance adalimumab achieved fistula healing. Table 3 summarises the differences in patients on adalimumab with and without fistula healing. Patients who achieved fistula healing had higher adalimumab trough levels compared to those who did not [9.2 (6.5-12.0) vs 5.4 (2.5-8.3) mg/L, P = 0.004]. Patients who achieved fistula healing had higher rates of combination therapy with an immunomodulator than those who did not (P = 0.048). The CRP and albumin levels were not significantly different in patients with and without fistula healing. ROC curve analysis identified a positive correlation between adalimumab trough levels and healing (AUC = 0.79, 95%CI: 0.66-0.93, P = 0.004) with an adalimumab trough level of 7.05 mg/L that maximised the sensitivity and specificity of infliximab levels in predicting fistula healing (sensitivity 70%; specificity 73%; OR = 6.3; P = 0.016; Figure 1B). Upon tertile analysis, higher tertiles of adalimumab levels were associated with a higher proportion of patients achieving fistula healing, with 62.5% healing rate for tertile 1 compared to 100% for tertile 3 (Figure 2B; P = 0.034). Out of the patients who achieved fistula healing on adalimu
| Patients with fistula healing (n = 37) | Patients without fistula healing (n = 11) | P value | |
| Median age, yr (IQR) | 33.0 (28.5-52.0) | 44.9 (33.0-52.0) | 0.254 |
| Female gender, n (%) | 10 (27.0) | 4 (36.4) | 0.550 |
| Mean weight, kg (SD) | 82.5 (22.3) | 79.7 (16.7) | 0.812 |
| Mean BMI, kg/m2 (SD) | 29.2 (5.9) | 25.5 (2.7) | 0.241 |
| Median age at diagnosis of Crohn’s disease (IQR) | 24.0 (18.0-42.0) | 30.0 (19.0-41.0) | 0.570 |
| A1, n (%) | 6 (16.2) | 1 (9.1) | - |
| A2, n (%) | 19 (51.4) | 7 (63.6) | - |
| A3, n (%) | 10 (27.0) | 3 (27.3) | - |
| Current smoker, n (%) | 5 (13.5) | 0 (0.0) | 0.198 |
| Location | |||
| Ileal, n (%) | 12 (32.4) | 5 (45.5) | - |
| Colonic, n (%) | 5 (13.5) | 2 (18.2) | - |
| Ileocolonic, n (%) | 15 (40.5) | 3 (27.3) | - |
| No luminal disease, n (%) | 2 (5.4) | 0 (0.0) | - |
| Upper gastrointestinal involvement, n (%) | 2 (5.4) | 1 (9.1) | - |
| Stricturing, n (%) | 9 (24.3) | 2 (18.2) | 0.644 |
| Penetrating, n (%) | 14 (37.8) | 3 (27.3) | 0.481 |
| Median duration on anti-TNF agent, wk (IQR) | 194.5 (124.3-311.3) | 122.5 (79.8-319.3) | 0.318 |
| Dose escalated anti-TNF therapy, n (%) | 10 (27.0) | 1 (9.1) | - |
| Concurrent steroids, n (%) | 0 (0.0) | 0 (0.0) | - |
| Concurrent aminosalicylates, n (%) | 4 (10.8) | 1 (9.1) | 0.849 |
| Combination with immunomodulator, n (%) | 25 (67.6) | 4 (36.4) | 0.048 |
| Methotrexate, n (%) | 7 (18.9) | 1 (9.1) | 0.424 |
| 6-mercaptopurine, n (%) | 6 (16.2) | 1 (9.1) | 0.537 |
| Azathioprine, n (%) | 12 (32.4) | 2 (18.2) | 0.336 |
| Concurrent allopurinol, n (%) | 4 (10.8) | 0 (0.0) | 0.248 |
| Mean albumin, g/L (SD) | 40.5 (4.5) | 40.0 (5.7) | 0.608 |
| Median CRP, mg/L (IQR) | 2.1 (1.0-4.5) | 5.4 (1.7-9.3) | 0.070 |
| Median trough level (IQR) | 9.2 (6.5-12.0) | 5.4 (2.5-8.3) | 0.004 |
| Detectable antibody, n (%) | 1 (2.7) | 1 (9.1) | 0.352 |
Fistulising perianal CD is a highly morbid condition for which treatment outcomes remain suboptimal in many patients. While there is limited data on the role of newer biologic agents such as ustekinumab in perianal CD[19], anti-TNF agents remain the treatment of choice. Our study showed a significant association between both infliximab and adalimumab trough levels and fistula healing, with higher levels associated with increased healing rates. We demonstrated that higher tertiles of both infliximab and adalimumab levels were associated with a higher proportion of patients achieving fistula healing. Notably, when plotting the cumulative percentage of healed patients against infliximab level, we found that 50% of the patients who achieve healing will heal with a level of 6.4 mg/L, 90% of the patients who achieve healing will heal with a level of 12.7 mg/L and 95% of the patients who achieve healing will heal with a level of 14.4 mg/L. Similarly, for patients on adalimumab, 50% of the patients who achieve healing will heal with a level of 9.2 mg/L, and 90% and 95% of patients who achieved fistula healing were healed with levels of 12.0 and 18.0 mg/L respectively. Our results support dose-escalation of both infliximab and adalimumab in non-responders, targeting higher levels to achieve fistula healing prior to changing biologic therapy. Importantly, this study is the largest study to date assessing the relationship between adalimumab trough levels and clinical fistula healing. This data adds to the growing body of evidence that fistula healing improves with higher anti-TNF trough levels, and that higher levels may be required for perianal fistula healing than for mucosal healing in luminal CD[12-14,20].
This study did not show an association between infliximab and adalimumab trough levels and fistula closure. Not all previous studies have assessed fistula closure, but some have found that patients with fistula closure had significantly higher maintenance infliximab and adalimumab trough levels[13,14]. Our results may have been limited by inadequate power due to relatively small numbers of patients who achieved fistula closure in our cohort. We had a high fistula healing rate in this study, with 72.7% and 77% of patients on maintenance infliximab and adalimumab achieving fistula healing respectively. This finding was possibly due to high rates of combination therapy with an immunomodulator (69.7% and 60.4% in the infliximab and adalimumab groups respectively).
Randomised controlled trials have shown that infliximab is effective at both inducing and maintaining fistula healing[7,8]. Our study found that fistula healing was associated with higher infliximab trough levels. This finding is supported by a post-hoc analysis of ACCENT II which found that higher infliximab trough levels during induction were associated with a complete absence of draining fistulas at week 14[12], as well as similar findings in other studies assessing induction and maintenance infliximab therapy[11,13]. In the future, there may be a role for the infliximab biosimilar CT-P13 in order to achieve these high infliximab levels required for perianal fistula healing; with recent randomised controlled trials demonstrating higher trough levels from subcutaneous administration of CT-P13 compared to intravenous administration[21]. Interestingly, our study found that fistula healing was associated with younger age in both univariate and multivariate analyses. Whilst patient factors including albumin and body weight have previously been shown to affect infliximab trough levels[22], the influence of age is unclear. This finding may be due to the relatively younger age at diagnosis of CD for patients with fistula healing or longer duration of infliximab therapy. Five patients in this study had been changed from infliximab to adalimumab or vice versa and were included in both groups, however the anti-TNF level and anti-TNF antibody levels at the time of changing treatment were not collected. Reassuringly, previous studies have demonstrated that the presence of infliximab antibodies does not decrease future response rates to adalimumab and vice versa[23].
Adalimumab has also been shown to be effective in both inducing[9] and maintaining fistula healing[24]. Our study found that fistula healing was associated with higher adalimumab trough levels. Whilst there is limited data on the association between adalimumab trough levels and fistula healing, our findings are consistent with two smaller retrospective studies that showed that patients with fistula healing had higher adalimumab trough levels compared to those without fistula healing[14,20]. On multivariate logistic regression analysis, adalimumab trough levels ≥ 7.05 mg/L and concurrent immunomodulator therapy both remained significantly associated with healing. This reflects how concomitant immunosuppressive therapy can be used to decrease the immunogenic response and therefore improve fistula healing rates[25].
This study has several limitations. Assessment of fistula healing was based on clinical assessment, which may not be as accurate as an objective assessment such as with magnetic resonance imaging of the pelvis. A recent study has demonstrated that higher anti-TNF trough levels are associated with improved rates of radiological healing in perianal fistulising CD[26]. However, the absence of drainage remains a clinically relevant endpoint that impacts on patient quality of life. In order to provide an objective marker of response, biochemical markers of disease activity including CRP and albumin were analysed and found not to correlate with fistula healing. Data was retrospectively collected, so in order to address this we only included patients with documented perianal exams and definitions for fistula healing and closure that were in line with previous randomised controlled trials[8]. We found that fistula healing is associated with higher infliximab and adalimumab trough levels, however further randomised controlled trials are required to assess whether dose escalation to higher levels improves healing and the optimal method for dose escalation. Whilst reactive TDM with dose escalation at the time of loss of response is effective, it remains unknown whether proactive TDM with subsequent dose modification improves outcomes. Notably, all previous studies on proactive TDM have focused on luminal disease with no prospective studies evaluating proactive TDM in perianal fistulising CD.
Our study showed that higher infliximab and adalimumab trough levels are associated with perianal CD fistula healing, with higher rates of healing in higher tertiles of infliximab and adalimumab levels. However, no association with fistula closure was observed. Further prospective studies are required to confirm target infliximab and adalimumab trough levels and determine the optimal dose escalation method to achieve these target levels.
Anti-tumor necrosis factor (anti-TNF)-alpha agents, including infliximab and adalimumab, are effective medical treatments for perianal fistulising Crohn’s disease (CD), but not all patients achieve fistula healing with up to 60% of patients treated with maintenance infliximab lose response within one year.
Accumulating evidence suggests that this loss of response is partly due to sub-therapeutic anti-TNF trough levels. Retrospective studies and post-hoc analyses of prospective data have identified that higher infliximab trough levels are associated with fistula healing and closure compared to what is observed for mucosal healing in luminal disease, with emerging data suggesting similar results for adalimumab. Quantitative assays for therapeutic drug monitoring permits individualisation of infliximab and adalimumab dosing, however there are very few studies on perianal fistulising CD and the optimal target levels for perianal fistulising CD remains unclear.
This study aims to assess the association between serum trough infliximab and adalimumab levels and perianal fistula healing and closure and identify optimal target levels.
In this multi-centre retrospective study conducted across four tertiary inflammatory bowel disease centres in Australia, we identified CD patients with perianal fistulae on maintenance infliximab or adalimumab who had a trough level within twelve weeks of clinical assessment. The primary outcome was fistula healing, defined as cessation in fistula drainage. The secondary outcome was fistula closure, defined as healing and closure of all external fistula openings. Differences between patients who did or did not achieve fistula healing were compared using the Chi-square test, t-test or Mann-Whitney U test.
Out of a total of 114 patients (66 infliximab, 48 adalimumab), 48 (72.7%) patients and 37 (77%) patients on maintenance infliximab and adalimumab respectively achieved fistula healing. Patients who achieved fistula healing had significantly higher infliximab and adalimumab trough levels compared to patients who did not [infliximab: 6.4 (3.8-9.5) vs 3.0 (0.3-6.2) mg/L, P = 0.003; adalimumab: 9.2 (6.5-12.0) vs 5.4 (2.5-8.3) mg/L, P = 0.004]. Serum trough levels for patients with and without fistula closure were not significantly different for infliximab [6.9 (4.3-10.2) vs 5.5 (2.5-8.3) mg/L, P = 0.105] or adalimumab [10.0 (6.6-12.0) vs 7.8 (4.2-10.0) mg/L, P = 0.083].
Higher maintenance infliximab and adalimumab trough levels are associated with perianal fistula healing in CD.
Our study showed that higher infliximab and adalimumab trough levels are associated with perianal CD fistula healing, with higher rates of healing in higher tertiles of infliximab and adalimumab levels, but no association with fistula closure was observed. Further prospective studies are required to confirm target infliximab and adalimumab trough levels and determine the optimal dose escalation method to achieve these target levels.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leal RF, Brazil; Triantafillidis J, Greece; Velikova TV, Bulgaria S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Makowiec F, Jehle EC, Starlinger M. Clinical course of perianal fistulas in Crohn's disease. Gut. 1995;37:696-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Lockhart-Mummery HE. Symposium. Crohn's disease: anal lesions. Dis Colon Rectum. 1975;18:200-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 89] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ramos A, Calvet X, Sicilia B, Vergara M, Figuerola A, Motos J, Sastre A, Villoria A, Gomollón F. IBD-related work disability in the community: Prevalence, severity and predictive factors. A cross-sectional study. United European Gastroenterol J. 2015;3:335-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Vollebregt PF, van Bodegraven AA, Markus-de Kwaadsteniet TML, van der Horst D, Felt-Bersma RJF. Impacts of perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:1253-1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 6. | Chaparro M, Zanotti C, Burgueño P, Vera I, Bermejo F, Marín-Jiménez I, Yela C, López P, Martín MD, Taxonera C, Botella B, Pajares R, Ponferrada A, Calvo M, Algaba A, Pérez L, Casis B, Maté J, Orofino J, Lara N, García-Losa M, Badia X, Gisbert JP. Health care costs of complex perianal fistula in Crohn's disease. Dig Dis Sci. 2013;58:3400-3406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1969] [Cited by in F6Publishing: 1782] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 8. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1581] [Cited by in F6Publishing: 1476] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 9. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1598] [Cited by in F6Publishing: 1526] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 10. | Colombel JF, Schwartz DA, Sandborn WJ, Kamm MA, D'Haens G, Rutgeerts P, Enns R, Panaccione R, Schreiber S, Li J, Kent JD, Lomax KG, Pollack PF. Adalimumab for the treatment of fistulas in patients with Crohn's disease. Gut. 2009;58:940-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 11. | Davidov Y, Ungar B, Bar-Yoseph H, Carter D, Haj-Natour O, Yavzori M, Chowers Y, Eliakim R, Ben-Horin S, Kopylov U. Association of Induction Infliximab Levels With Clinical Response in Perianal Crohn's Disease. J Crohns Colitis. 2017;11:549-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Papamichael K, Vande Casteele N, Jeyarajah J, Osterman M, Cheifetz A. Adequate infliximab exposure during the induction phase is associated with early complete fistula response in patients with fistulizing Crohn’s disease: a post-hoc analysis of the Accent-2 trial. Gastroenterology. 2019;156:S-111. [DOI] [Cited in This Article: ] |
| 13. | Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, Patel A, Best K, Fox C, Idstein K, Abreu MT. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Ther. 2017;45:933-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 191] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 14. | Plevris N, Jenkinson PW, Arnott ID, Jones GR, Lees CW. Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn's disease. Eur J Gastroenterol Hepatol. 2020;32:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1523] [Cited by in F6Publishing: 1453] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 16. | Bortlik M, Duricova D, Malickova K, Machkova N, Bouzkova E, Hrdlicka L, Komarek A, Lukas M. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013;7:736-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Australian Government Department of Health. Crohn disease: infliximab, adalimumab and vedolizumab, Drug utilisation sub-committee (DUSC). [cited 14 August 2021]. Available from: https://www.pbs.gov.au/pbs/industry/listing/participants/public-release-docs/2017-06/crohn-disease-infliximab-adalimumab-vedolizumab-prd-2017-06. [Cited in This Article: ] |
| 18. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1970] [Cited by in F6Publishing: 2102] [Article Influence: 116.8] [Reference Citation Analysis (2)] |
| 19. | Chapuis-Biron C, Kirchgesner J, Pariente B, Bouhnik Y, Amiot A, Viennot S, Serrero M, Fumery M, Allez M, Siproudhis L, Buisson A, Pineton de Chambrun G, Abitbol V, Nancey S, Caillo L, Plastaras L, Savoye G, Chanteloup E, Simon M, Dib N, Rajca S, Amil M, Parmentier AL, Peyrin-Biroulet L, Vuitton L; GETAID BioLAP Study Group. Ustekinumab for Perianal Crohn's Disease: The BioLAP Multicenter Study From the GETAID. Am J Gastroenterol. 2020;115:1812-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Strik AS, Löwenberg M, Buskens CJ, B Gecse K, I Ponsioen C, Bemelman WA, D'Haens GR. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn's disease patients. Scand J Gastroenterol. 2019;54:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Westhovens R, Yoo DH, Jaworski J, Matyska-Piekarska E, Smiyan S, Ivanova D, Zielinska A, Raussi E, Batalov A, Lee SJ, LeeSY, Suh JH. THU0191 Novel formulation of ct-p13 for subcutaneous administration in patients with rheumatoid arthritis: initial results from a phase i/iii randomised controlled trial. Ann Rheum Dis. 2018;77:315. [DOI] [Cited in This Article: ] |
| 22. | Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247-2259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, Claes K, Coopman T, Van Schuerbeek N, Van Assche G, Vermeire S, Rutgeerts P. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 381] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Panaccione R, Colombel JF, Sandborn WJ, D'Haens G, Zhou Q, Pollack PF, Thakkar RB, Robinson AM. Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236-1247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2221] [Cited by in F6Publishing: 2214] [Article Influence: 158.1] [Reference Citation Analysis (0)] |
| 26. | De Gregorio M, Lee T, Krishnaprasad K, Amos G, An YK, Bastian-Jordan M, Begun J, Borok N, Brown DJM, Cheung W, Connor SJ, Gerstenmaier J, Gilbert LE, Gilmore R, Gu B, Kutaiba N, Lee A, Mahy G, Srinivasan A, Thin L, Thompson AJ, Welman CJ, Yong EXZ, De Cruz P, van Langenberg D, Sparrow MP, Ding NS. Higher Anti-tumor Necrosis Factor-α Levels Correlate With Improved Radiologic Outcomes in Crohn's Perianal Fistulas. Clin Gastroenterol Hepatol. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |