Copyright
©The Author(s) 2022.
World J Gastroenterol. May 28, 2022; 28(20): 2214-2226
Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2214
Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2214
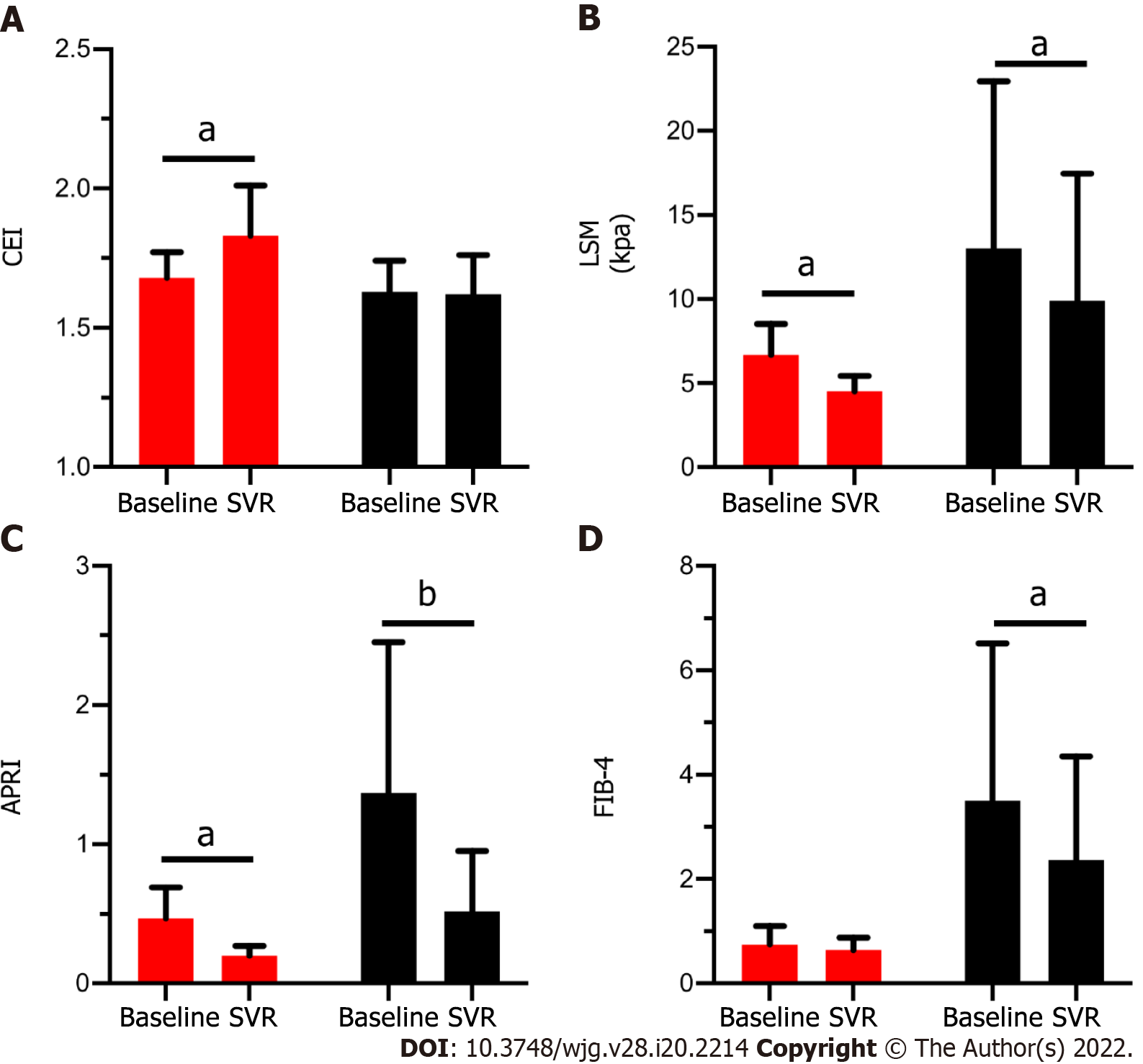
Figure 3 Comparison of four noninvasive methods before and after sustained virological response between patients with fibrosis regression or not.
A: Value of contrast enhancement index; B: Value of liver stiffness measurement; C: Value of aspartate aminotransferase-to-platelet ratio index; D: Value or Fibrosis-4 in patients with (Red column) and without (Black column) fibrosis regression at baseline and after achieving sustained virological response (SVR). Red column: Patients had fibrosis regression after achieving SVR (n = 7). Black column: Patients didn’t have fibrosis regression after achieving SVR (n = 14). aP < 0.05; bP < 0.001. CEI: Contrast enhancement index; SVR: Sustained virological response; LSM: Liver stiffness measurement; APRI: Aspartate aminotransferase-to-platelet ratio index; FIB-4: Fibrosis-4.
- Citation: Li XH, Huang R, Yang M, Wang J, Gao YH, Jin Q, Ma DL, Wei L, Rao HY. Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced magnetic resonance imaging for evaluating fibrosis regression in chronic hepatitis C patients after direct-acting antiviral. World J Gastroenterol 2022; 28(20): 2214-2226
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2214