Copyright
©The Author(s) 2022.
World J Gastroenterol. May 7, 2022; 28(17): 1725-1750
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1725
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1725
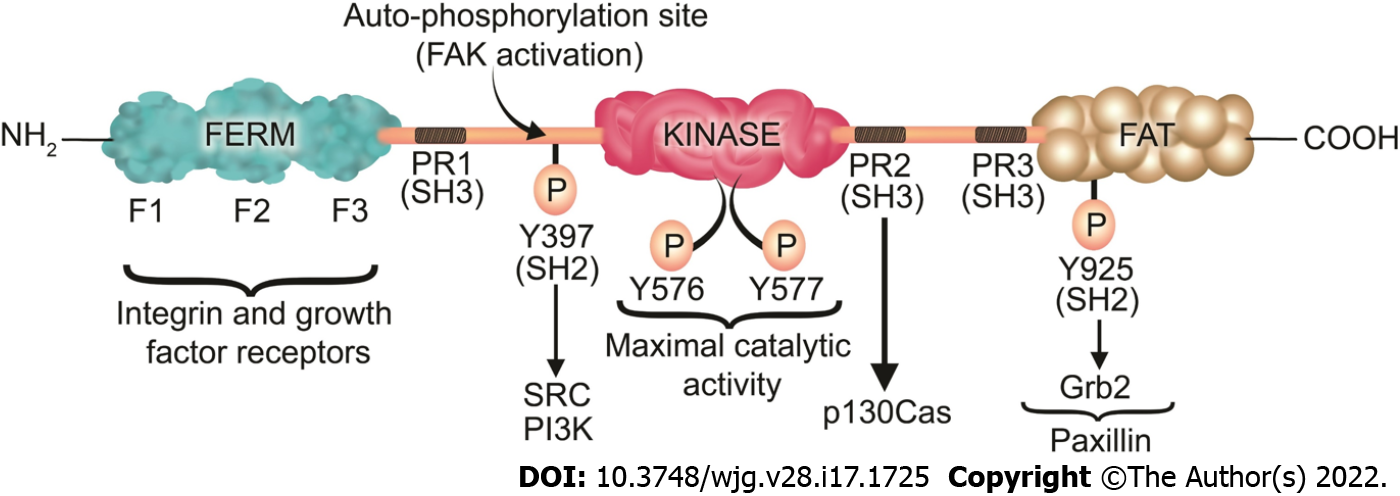
Figure 3 Focal adhesion kinase structure, phosphorylation sites, and its associated proteins.
Focal adhesion kinase (FAK) contains an N-terminal band 4.1-ezrin-radixin-moesin (FERM) domain comprised of three lobes (F1, F2, and F3), a central kinase domain, a C-terminal FAT domain, and two linker domains with three PR regions that bind SH3 domain containing protein such as p130Cas. Y397 is the site of the FAK autophosphorylation, crucial for FAK activation, which interacts with proteins containing the SH2 domain such as Src and PI3K. Subsequently to the SH2 binding, Src binds to the PR1 SH3 domain (PXXP) and further phosphorylates the Y576/577 sites on FAK, which are crucial for the maximal catalytic activity of FAK. Further FAK phosphorylation at Y925 creates a binding site for Grb2. The phosphorylation of FAK-Y-925 and subsequent Grb2 binding disassociates paxillin from FAK, which results in FAK release from FAs, thus stimulating FA disassembly. The FERM domain regulates the interactions of FAK with growth factor receptors and integrins. The FAT domain recruits FAK to FAs by associating with paxillin. FERM: Band 4.1-ezrin-radixin-moesin; FAT: Focal adhesion targeting; PR: Proline-rich region; SH: Src homology; P: Phosphorylation.
- Citation: Oncel S, Basson MD. Gut homeostasis, injury, and healing: New therapeutic targets. World J Gastroenterol 2022; 28(17): 1725-1750
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1725