Copyright
©The Author(s) 2021.
World J Gastroenterol. Mar 7, 2021; 27(9): 886-907
Published online Mar 7, 2021. doi: 10.3748/wjg.v27.i9.886
Published online Mar 7, 2021. doi: 10.3748/wjg.v27.i9.886
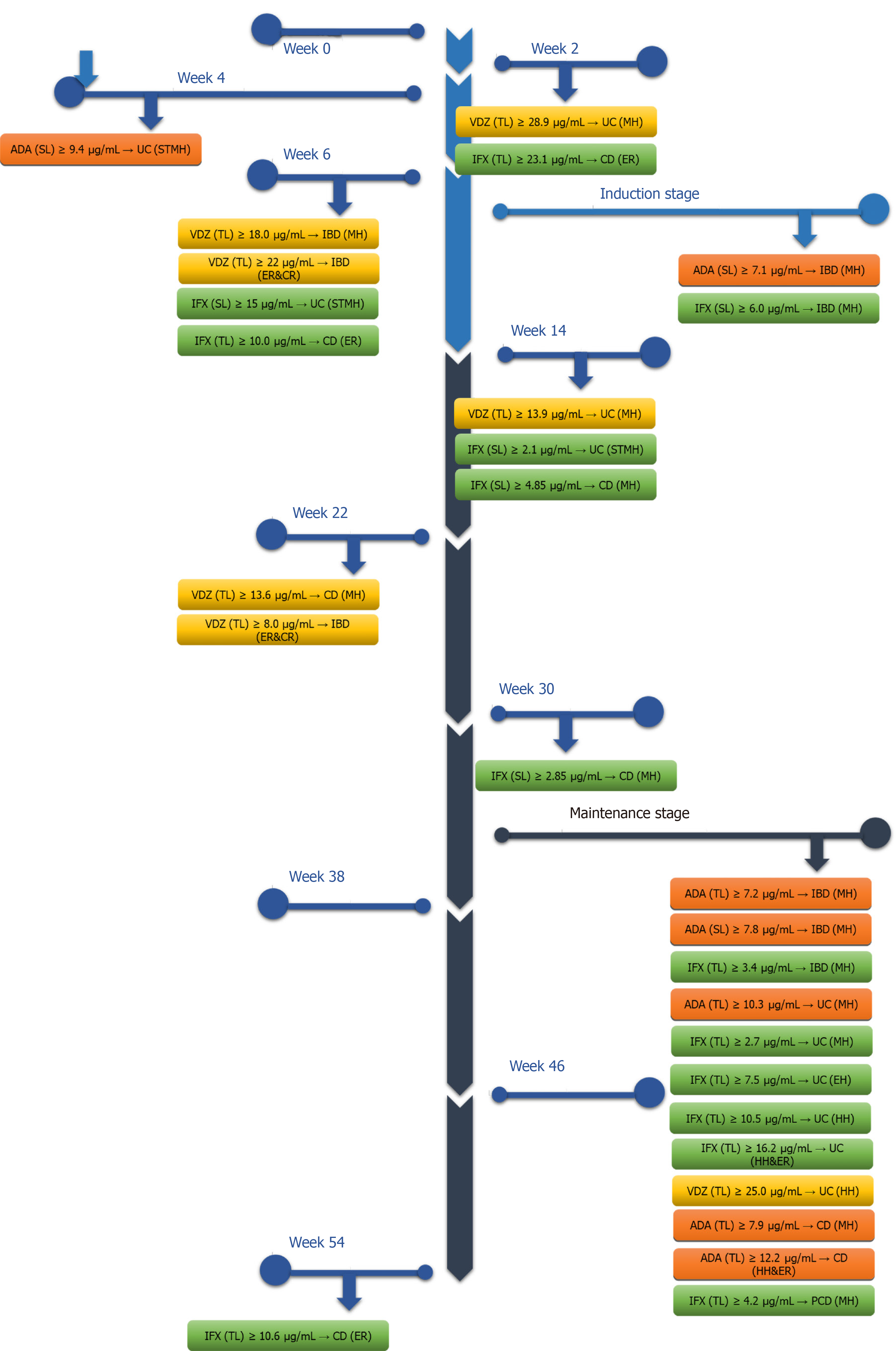
Figure 2 Target of blood concentration during different therapeutic stages of biologics.
IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; PCD: Pediatric Crohn’s disease; IFX: Infliximab; ADA: Adalimumab; VDZ: Vedolizumab; MH: Mucosal healing; HH: Histological healing; EH: Endoscopic healing; ER: Endoscopic remission; CR: Clinical remission; STMH: Short term mucosal healing; SL: Serum level; TL: Trough level.
- Citation: Cao WT, Huang R, Jiang KF, Qiao XH, Wang JJ, Fan YH, Xu Y. Predictive value of blood concentration of biologics on endoscopic inactivity in inflammatory bowel disease: A systematic review. World J Gastroenterol 2021; 27(9): 886-907
- URL: https://www.wjgnet.com/1007-9327/full/v27/i9/886.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i9.886