Copyright
©The Author(s) 2021.
World J Gastroenterol. Aug 28, 2021; 27(32): 5362-5375
Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5362
Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5362
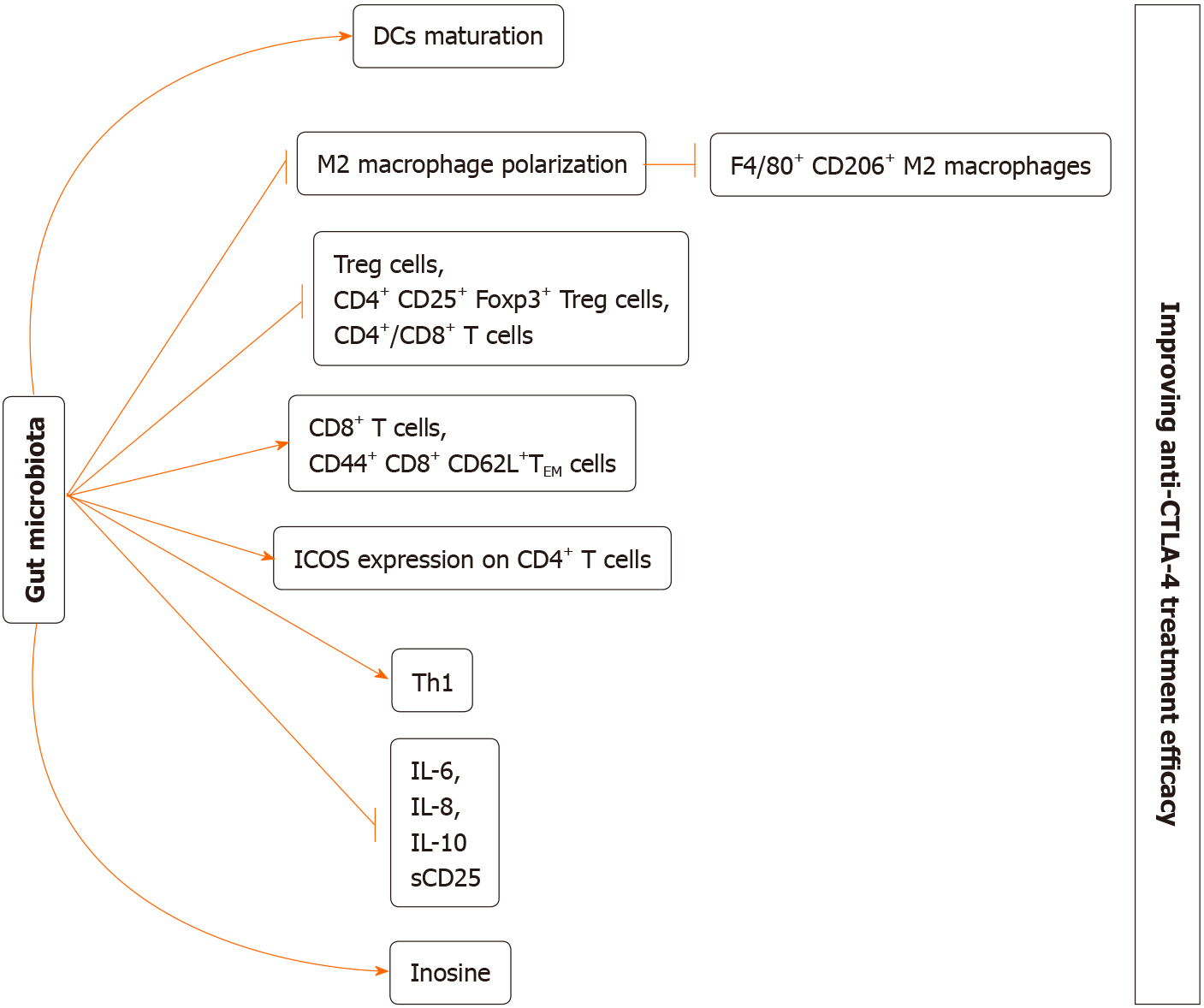
Figure 2 The potential mechanism of gut microbiome regulating anti-cytotoxic T-lymphocyte-associated protein 44 treatment efficacy.
(1) Gut microbiota may induce dendritic cells maturation; (2) Gut microbiota may inhibit the M2 polarization, thereby decreasing M2 macrophages (F4/80+CD206+); (3) Gut microbiota may decrease regulatory T cells, CD4+ CD25+ Foxp3+ regulatory T cells, and CD4+/CD8+ T cells; (4) Gut microbiota increase CD8+ T cells and CD44+CD8+CD62L+ effector memory T cells; (5) Gut microbiota may increase inducible T cell co-stimulator expression on CD4+ T cells; (6) Gut microbiota may induce T helper 1 immune response; (7) Gut microbiota may reduce interleukin (IL)-6; IL-8; IL-10, and sCD25 level; and (8) Gut microbiota may increase inosine production. Altogether, all of these approaches may eventually improve anti- cytotoxic T-lymphocyte-associated protein 4 treatment efficacy. DCs: Dendritic cells; Treg: Regulatory T; TEM: Effector memory T cells; ICOS: Inducible T cell co-stimulator; sCD25: Soluble CD25; IL: Interleukin.
- Citation: Kang YB, Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol 2021; 27(32): 5362-5375
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5362