Published online Jul 7, 2021. doi: 10.3748/wjg.v27.i25.3851
Peer-review started: December 27, 2020
First decision: January 23, 2021
Revised: January 27, 2021
Accepted: March 15, 2021
Article in press: March 15, 2021
Published online: July 7, 2021
Gastric cancer (GC) is a common malignancy that results in a high rate of cancer-related mortality. Cisplatin (DDP)-based chemotherapy is the first-line clinical treatment for GC therapy, but chemotherapy resistance remains a severe clinical challenge. Zinc oxide nanoparticle (ZnO-NP) has been identified as a promising anti-cancer agent, but the function of ZnO-NP in GC development is still unclear.
To explore the effect of ZnO-NP on chemotherapy resistance during GC pro
ZnO-NP was synthesized, and the effect and underlying mechanisms of ZnO-NP on the malignant progression and chemotherapy resistance of GC cells were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, colony formation assays, transwell assays, wound healing assays, flow cytometry, and Western blot analysis in GC cells and DDP-resistant GC cells, and by tumorigenicity analyses in nude mice.
Our data revealed that ZnO-NP was able to inhibit proliferation, migration, and invasion and induce apoptosis of GC cells. Meanwhile, ZnO-NP significantly reduced the half maximal inhibitory concentration (IC50) of DDP for the inhibition of cell proliferation of DDP-resistant SGC7901/DDP cell lines. Autophagy was increased in DDP-resistant GC cells, as demonstrated by elevated light chain 3-like protein 2 (LC3II)/LC3I and Beclin-1 expression and repressed p62 expression in SGC7901/DDP cells compared to SGC7901 cells. Mechanically, ZnO-NP inhibited autophagy in GC cells and treatment with DDP induced autophagy, which was reversed by ZnO-NP. Functionally, ZnO-NP attenuated the tumor growth of DDP-resistant GC cells in vivo.
We conclude that ZnO-NP alleviates the chemoresistance of GC cells by inhibiting autophagy. Our findings present novel insights into the mechanism by which ZnO-NP regulates the chemotherapy resistance of GC. ZnO-NP may serve as a potential therapeutic candidate for GC treatment. The potential role of ZnO-NP in the clinical treatment of GC needs clarification in future investigations.
Core Tip: We show that zinc oxide nanoparticle (ZnO-NP) reduces the chemoresistance of gastric cancer (GC) cells by inhibiting autophagy. Our findings provide innovative insights into the scenario in which ZnO-NP mediates chemotherapy resistance in GC. ZnO-NP may serve as a potential therapeutic candidate for GC treatment.
- Citation: Miao YH, Mao LP, Cai XJ, Mo XY, Zhu QQ, Yang FT, Wang MH. Zinc oxide nanoparticles reduce the chemoresistance of gastric cancer by inhibiting autophagy. World J Gastroenterol 2021; 27(25): 3851-3862
- URL: https://www.wjgnet.com/1007-9327/full/v27/i25/3851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i25.3851
Gastric cancer (GC) is the second most common cause of cancer-related mortality globally[1]. Chemotherapy is the preferred treatment for advanced-stage GC patients, in which oxaliplatin, 5-fluorouracil (5-FU), cisplatin (DDP) are first-line therapies[2-4]. Although advancements have been made in chemotherapy effectiveness, the survival rate remains unsatisfactory due to chemotherapy resistance[5,6], which significantly limits the efficiency of GC treatments[7]. The molecular mechanisms underlying the regulation of GC chemotherapy resistance are complicated and remain poorly understood[8]. Accordingly, therapeutic strategies for the attenuation of chemo
Autophagy is a process in which cellular contents, such as dysfunctional organelles and large protein groups, are transported to lysosomes for degradation and reuse[9]. Autophagy sustains cellular homeostasis and limits cellular damage under multiple stresses[10]. Autophagy has dual roles in cancer progression[11]. In some cases, autophagy induces cancer cell survival by recovering intracellular contents and increasing energy generation to reach the high metabolic requirements of cancer cells. In other contexts, autophagy inhibits cell imbalance and damage to attenuate tumorigenesis[12]. Autophagy is closely associated with the chemotherapy resistance of GC cells, and inhibition of autophagy relieves chemoresistance[13,14]. Autophagy is correlated with cell differentiation and tumor development in GC[15]. Thus, auto
Nanoparticles (NPs) are currently applied in multiple biomedical fields including bone regeneration, wound healing, bio-imaging, and targeted-drug transmission systems[17-21]. The conversion of material to nanoscale regularly leads to changes in chemical, physical (electric and magnetic), morphological, and structural properties[19,21]. These modifications permit NPs to cooperate with different biomolecules to affect certain responses[19,21]. Due to the unique surface and size properties, NPs have numerous benefits that enable them to serve as potential anti-tumor therapeutics[22]. Among them, metal zinc oxide NPs (ZnO-NP) have various properties and are widely used as critical components of many biomedical and cosmetic applications including sunscreen, foot care, and ointments[23]. ZnO-NPs exhibit antibacterial activities[24,25], and are also extensively used in drug targeting due to their biocompatibility[26]. However, the effect of ZnO-NP on the chemotherapy resistance of GC cells remains unknown.
In this study, we focused on the impact of ZnO-NP on chemotherapy resistance of GC cells. We revealed the innovative role of ZnO-NP in repressing chemoresistance and reducing GC progression via inhibition of autophagy.
The SGC7901, BGC823, and SGC7901/DDP cell lines were maintained in the lab. The cells were incubated in an incubator of 5% CO2 and 37 °C in RPMI 1640 medium (Hyclone, Logan, UT, United States) with fetal bovine serum (10%; Hyclone), streptomycin (0.1 mg/mL; Hyclone) and penicillin (100 units/mL; Hyclone). DDP was obtained (Sigma, St. Louis, MO, United States) and used at the indicated doses.
A total of 0.5 mol/L zinc nitrate was plated to 1 mol/L sodium hydroxide solution, followed by continuous stirring (15 min). The white precipitate formed was washed and centrifuged, followed by repeated distilling of H2O. The collected white powder [Zn(OH)2] was dried in a hot air oven at 60 °C. During drying, Zn2[24] was entirely converted to ZnO. Then, the dried ZnO powder was annealed at 500 °C for 3 h to convert to ZnO-NP. To analyze the effect of ZnO-NP on the malignant progression and chemotherapy resistance, the GC cells were treated with ZnO-NP at a dose of 5 μg/mL.
Cell viability was assessed by MTT assays at the indicated times in 6-well dishes. Briefly, the MTT solution (Solarbio, Beijing, China) was added to the cells and cultured at 5% CO2 and 37 °C for 4 h. Next, dimethyl sulfoxide (100 μL, 10 min; Sigma) was used to terminate the reaction. Cell viability was analyzed at an absorbance of 490 nm with a microplate reader (Thermo Fisher Scientific, Waltham, MA, United States).
About 1 × 103 cells were plated in 6-well dishes and cultured in RPMI 1640 medium at 5% CO2 and 37 °C. After 2 wk, cells were washed with phosphate-buffered saline (PBS) for about 30 min and dyed with 1% crystal violet dye, after which the number of colonies was calculated.
Transwell assays were conducted to analyze the invasion and migration of melanoma cells by using a Transwell plate (Corning, New York, NY, United States) according to the manufacturer’s instructions. Briefly, the upper chambers were plated with about 1 × 105 cells. Then cells were fixed in 4% paraformaldehyde and dyed with crystal violet. The invaded and migrated cells were recorded and calculated.
Cells were plated in a 24-well plate at a density of 3 × 105 cells/well and cultured overnight to reach full confluence as a monolayer. A 20 μL pipette tip was applied to slowly cut a straight line across the well. Then the well was washed three times with PBS, and changed to serum-free medium, followed by continued culture. The wound healing percentage was calculated.
Cell apoptosis was measured using the Annexin-V-Fluorescein Isothiocyanate Apoptosis kit (BD Biosciences, San Jose, CA, United States) based on flow cytometry analysis using the FACSCalibur flow cytometer, followed by quantification with FlowJo software.
Total proteins were extracted from the cells using radioimmunoprecipitation assay buffer (Cell Signaling Technology, Danvers, MA, United States) and quantified using the BCA Protein Quantification Kit (Abbkine Scientific Co., Ltd., Palo Alto, CA, United States). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, United States), followed by incubation with 5% milk and with primary antibodies at 4 °C overnight. The membranes were incubated with the corresponding secondary antibodies (Boster Biotechnology, Wuhan, China) for 1 h at room temperature, followed by protein detection by chemiluminescence (Beyotime Biotechnology, Shanghai, China). The primary antibodies used in this study were against light chain 3B (LC3B), p62, Beclin-1, and β-actin (all from Abcam, Cambridge, MA, United States).
The tumor growth of GC cells in vivo was evaluated in Balb/c nude mice (4-week-old, male, n = 5). About 1 × 107 SGC7901/DDP cells were subcutaneously injected in the mice. After 5 d, we measured tumor growth every 5 d. We sacrificed the mice after 30 d, and tumors were scaled. Tumor volume (V) was determined by estimating the length (L) and width (W) with calipers and measured with the formula (L × W2) × 0.5. Animal care and methods were authorized by the Animal Ethics Committee of Nantong Third People’s Hospital (Jiangsu, China).
Data are presented as mean ± SD, and statistical analyses were performed with GraphPad Prism 7. The unpaired Student’s t-test was applied for comparing two groups, and one-way analysis of variance was applied for comparing among multiple groups. aP < 0.05 was considered statistically significant.
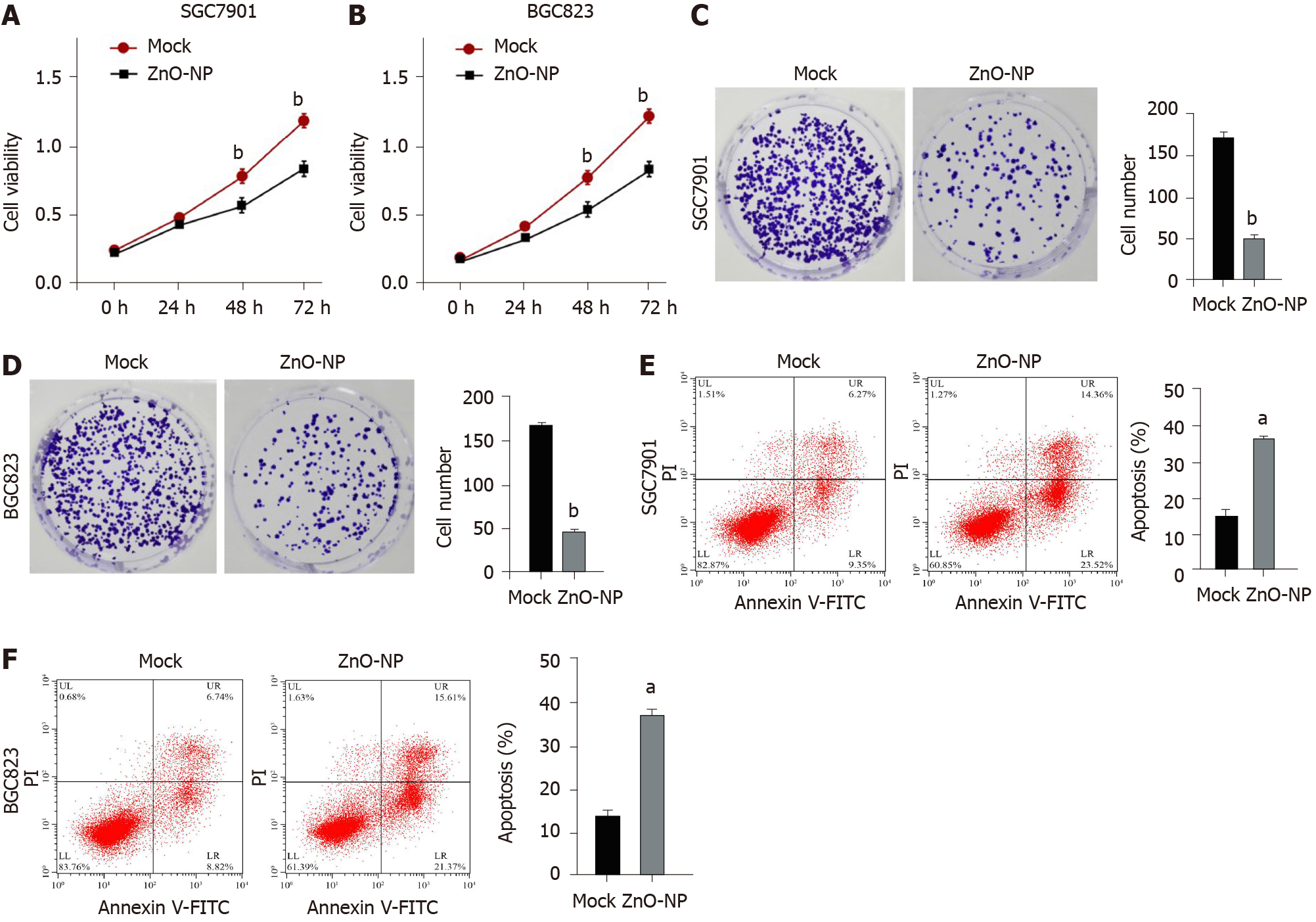
To investigate the effect of ZnO-NP on GC cells, SGC7901 and BGC823 cells were treated with ZnO-NP or an equal volume of saline. The cell viability was significantly inhibited by ZnO-NP treatment of the cells (Figure 1A and B). Consistently, ZnO-NP markedly reduced the colony numbers of SGC7901 and BGC823 cells (Figure 1C and D). Moreover, the apoptosis of SGC7901 and BGC823 cells was enhanced by treatment with ZnO-NP (Figure 1E and F), suggesting that ZnO-NP is able to inhibit proliferation and induce apoptosis of GC cells.
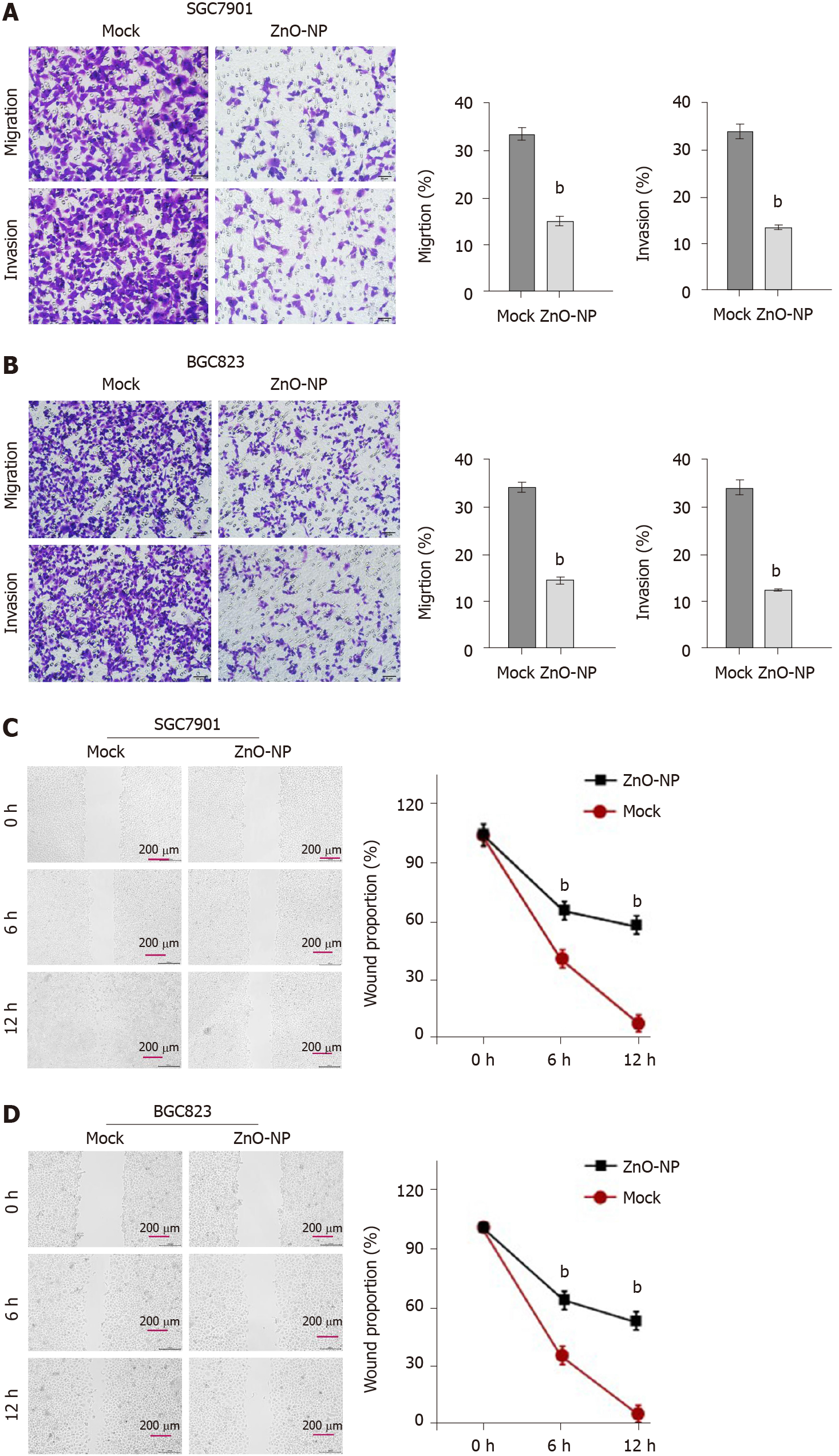
Next, the role of ZnO-NP in regulating the invasion and migration was evaluated. Transwell assays revealed that the invasion and migration of BGC823 and SGC7901 cells were significantly attenuated upon treatment with ZnO-NP (Figure 2A and B). In addition, wound healing assays demonstrated that ZnO-NP markedly enhanced the wound proportion in SGC7901 and BGC823 cells (Figure 2C and D), indicating that ZnO-NP alleviates the migration and invasion of GC cells.
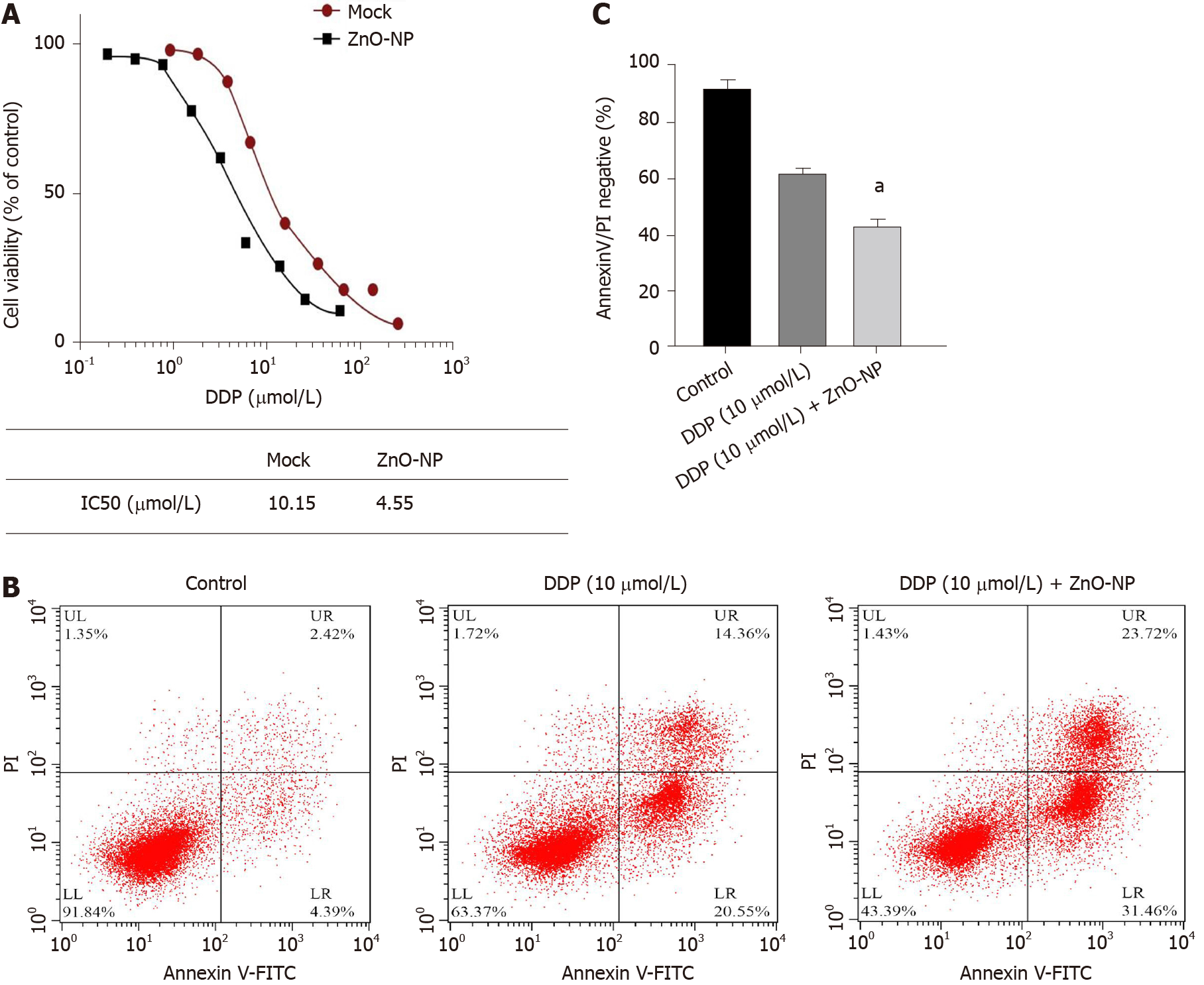
We further explored the impact of ZnO-NP on the DDP of GC cells. Significantly, treatment with ZnO-NP notably reduced the IC50 value of DDP for inhibition of cell proliferation in DDP-resistant SGC7901/DDP cell lines (Figure 3A). Furthermore, DDP enhanced the apoptosis of SGC7901/DDP cells, which was markedly reinforced by ZnO-NP treatment (Figure 3B and C), suggesting that ZnO-NP attenuates the DDP resistance of GC cells.
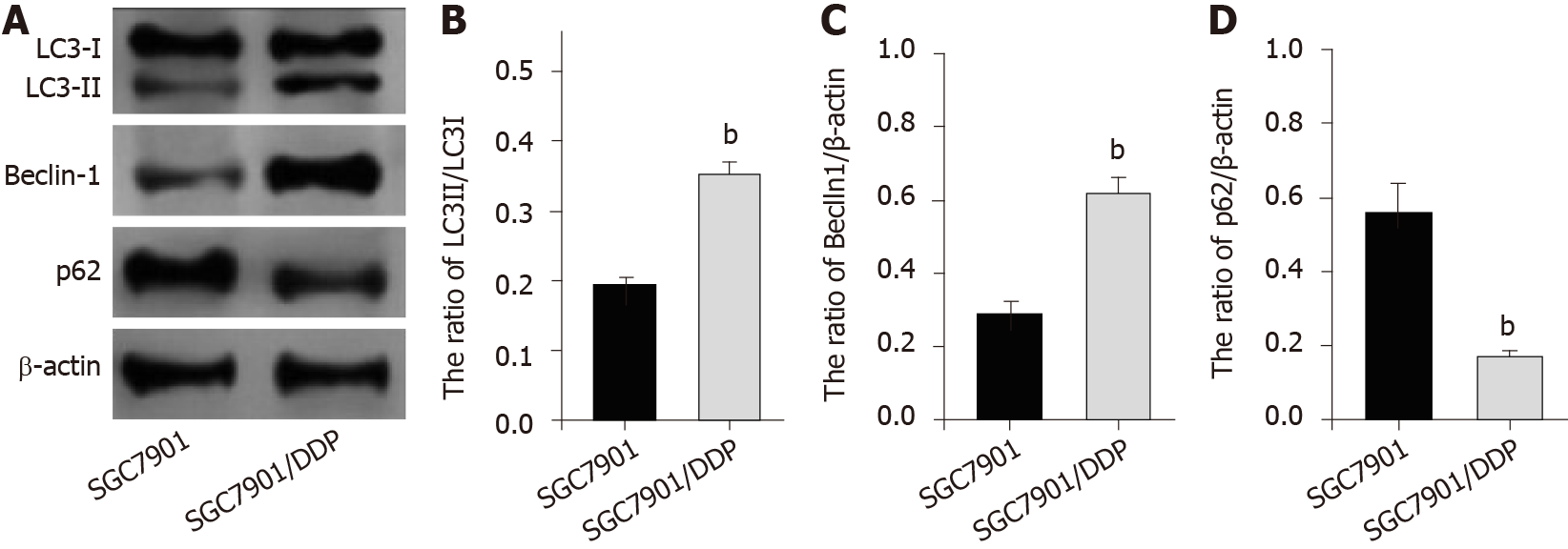
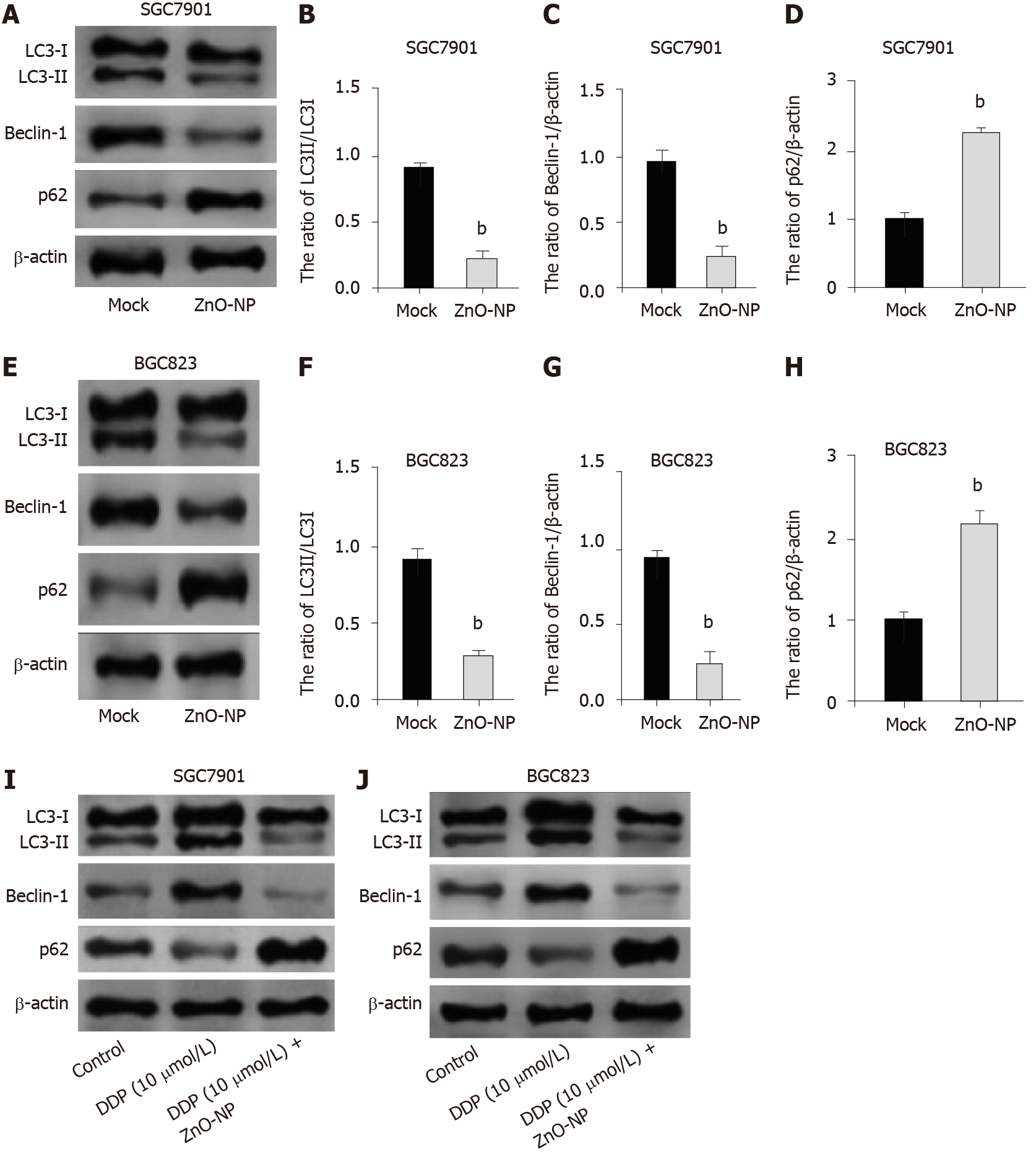
Next, we were interested in the correlation of autophagy with the DDP resistance of GC cells. For this purpose, we analyzed the expression of autophagy markers including LC3B-II, LC3B-I, Beclin-1, and p62 in SGC7901 and SGC7901/DDP cell lines. Our data showed that the expression ratio of LC3II/LC3I and levels of Beclin-1 were elevated while p62 expression was inhibited in SGC7901/DDP cells compared with those in SGC7901 cells (Figure 4), indicating that autophagy is increased in DDP-resistant GC cells.
We investigated the effect of ZnO-NP on autophagy in GC cells. We found that the treatment of ZnO-NP inhibited LC3II/LC3I and Beclin-1 levels but promoted p62 expression in SGC7901 and BGC823 cells (Figure 5A-H). Moreover, our data revealed that treatment with DDP induced the expression ratio of LC3II/LC3I and the levels of Beclin-1 and decreased the p62 expression in the cells; treatment with ZnO-NP reversed this effect (Figure 5I and J), indicating that ZnO-NP can inhibit autophagy in GC cells.
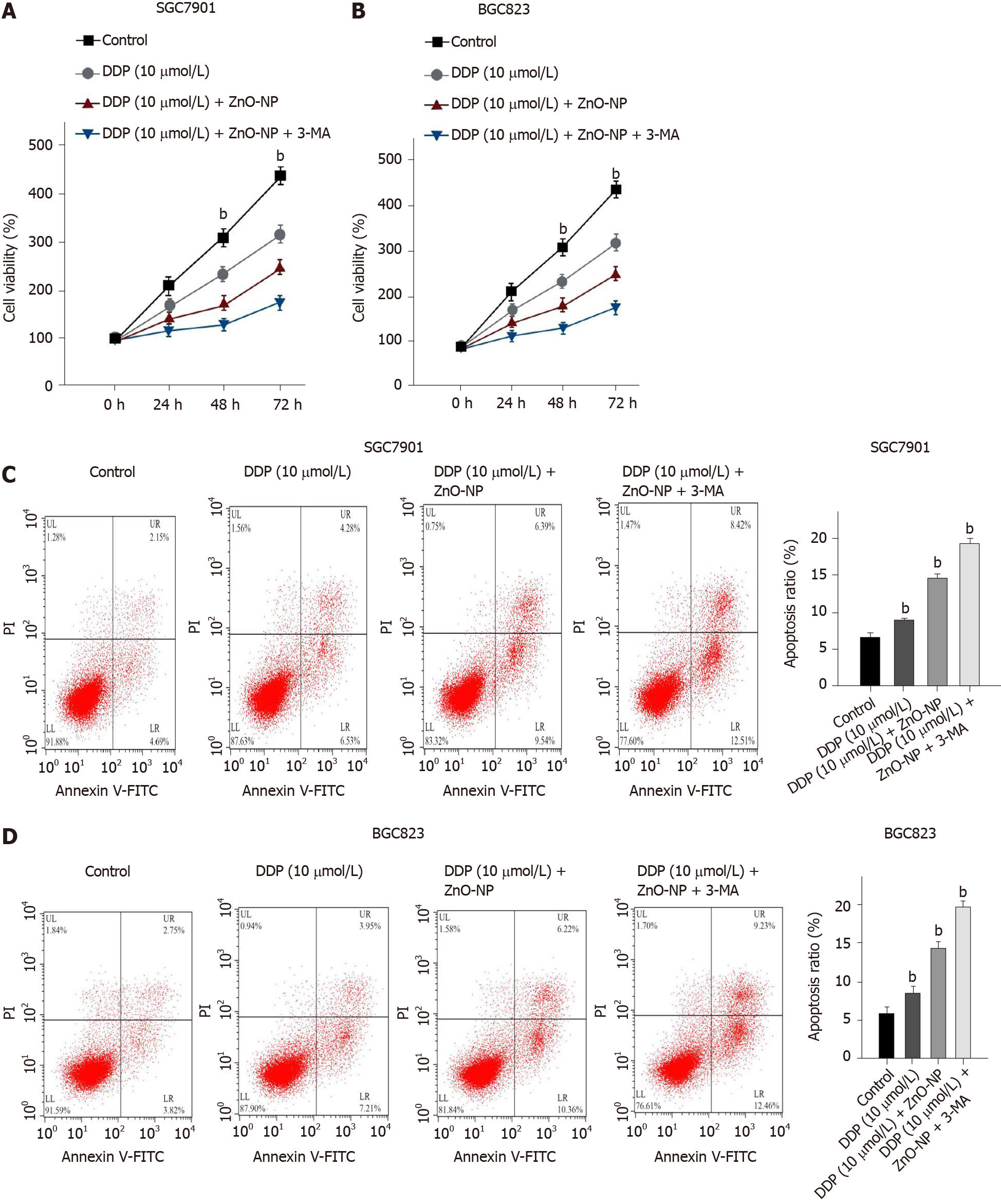
We explored whether ZnO-NP modulated the DDP resistance of GC cells by regulating autophagy. Treatment with DDP reduced the viability of SGC7901 and BGC823 cells, while ZnO-NP or the autophagy inhibitor 3-methyladenine (3-MA) was able to further inhibit the phenotype (Figure 6A and B). Moreover, the cell apoptosis of SGC7901 and BGC823 cell lines was induced by DDP treatment, in which the treatment of ZnO-NP or 3-MA could reverse this effect in the cells (Figure 6C and D), suggesting that ZnO-NP attenuates chemotherapy drug resistance by inhibiting autophagy of GC cells.
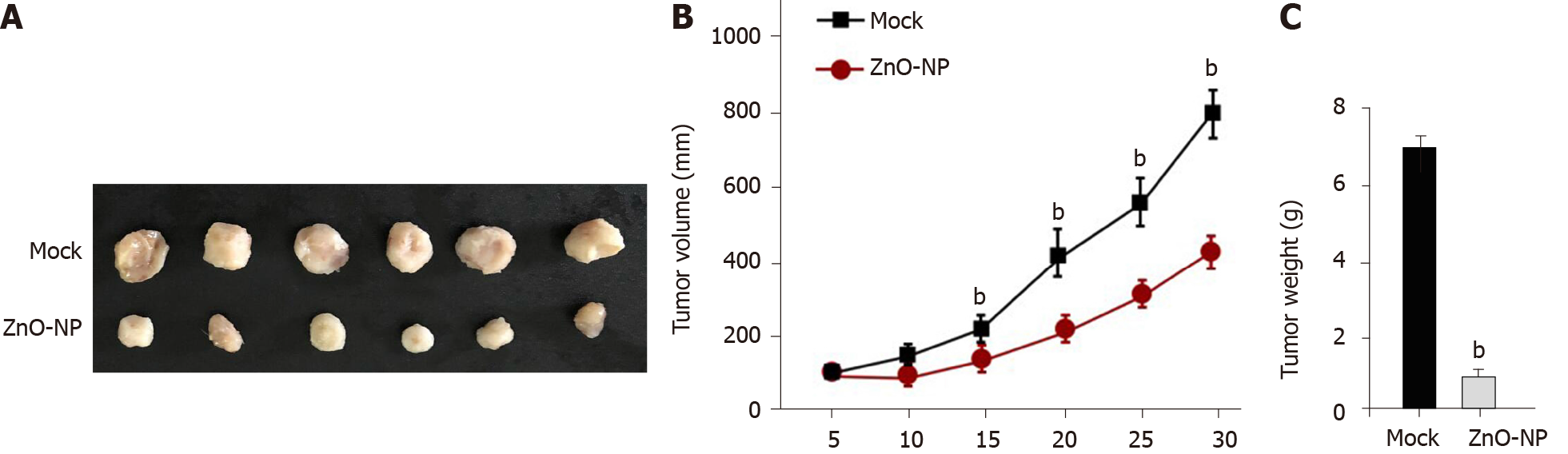
Next, the effect of ZnO-NP on DDP-resistant GC cell growth in vivo was assessed by tumorigenicity analysis. The tumor growth of SGC7901/DDP cells was attenuated by ZnO-NP treatment of nude mice (Figure 7), indicating that ZnO-NP is able to reduce the tumor growth of chemoresistant GC cells in vivo.
The chemotherapy resistance of GC patients serves as a severe clinical challenge[1]. ZnO-NP has potential anti-tumor activities, but its role in modulating the chemo
Previous studies have identified the cancer-inhibitory effect of ZnO-NP. ZnO-NP promotes proteotoxic and oxidative stress and induces the apoptosis of ovarian cancer cells in a p53-mutation-dependent manner[27]. ZnO-NP enhances the cell death of multiple human myelomas by regulating reactive oxygen species and cytochrome c/apoptotic protease activating factor 1/caspase-9 signaling[28]. ZnO-NP increases the apoptosis of human ovarian cancer cells[29]. Frizzled-7-targeted delivery of ZnO-NP induces inhibitory effects on the drug resistance of breast cancer cells[30]. Moreover, iron NPs reverse the chemotherapy resistance of GC cells[31]. In this study, we found that ZnO-NP inhibited proliferation, migration, and invasion and induced apoptosis of GC cells. Meanwhile, ZnO-NP attenuated the DDP resistance of GC cells. Moreover, ZnO-NP was able to repress the tumor growth of chemoresistance GC cells in vivo. Our findings indicate the innovative effect of ZnO-NP on the chemotherapy drug resistance of GC cells, demonstrating critical evidence of metal oxide NPs in the regulation of cancer development.
Furthermore, previous studies have identified that autophagy is clearly correlated with chemotherapy drug resistance and the development of GC, and targeting autophagy is involved in the modulation of chemoresistance GC cells. Long noncoding RNA MALAT1 modulates autophagy-related chemoresistance by targeting miR-23b-3p in GC[14]. Autophagy contributes to the chemoresistance of GC stem cells by regulating Notch signaling[32]. Tripartite motif containing 14 induces autophagy and chemotherapy resistance of GC cells via modulating adenosine monophosphate-activated protein kinase/mechanistic target of rapamycin (mTOR) signaling[33]. Cluster of differentiation 133 (CD133) inhibition reduces DDP resistance by repressing autophagy and phosphatidylinositol 3-kinase/AKT/mTOR signaling in CD133-positive GC cells[34].
CDGSH iron sulfur domain 2 improves the chemosensitivity of GC cells by en
In summary, we conclude that ZnO-NP reduces the chemoresistance of GC cells by inhibiting autophagy. Our findings provide innovative insights into the scenario in which ZnO-NP mediates the chemotherapy resistance of GC. ZnO-NP may serve as a potential therapeutic candidate for GC treatment. The potential role of ZnO-NP in the clinical treatment of GC needs to be clarified in future investigations.
Gastric cancer (GC) is a common cancer and results in a high rate of tumor-related mortality. Cisplatin (DDP)-based chemotherapy is the first-line treatment of GC, but chemoresistance remains a severe clinical problem. Zinc oxide nanoparticle (ZnO-NP) has been identified as a promising anti-cancer agent, but its role in GC development is still unclear.
To identify the role of ZnO-NP in the regulation of GC progression.
This study explored the effect of ZnO-NP on chemotherapy resistance during GC progression.
ZnO-NP was synthesized, and the effect and underlying mechanism on the malignant progression and chemotherapy resistance of GC cells were assessed by tumorigenicity in nude mice and evaluated by Western blotting, flow cytometry analysis, wound healing assays, transwell assays, colony formation assays, and MTT assays in GC cells and DDP-resistant GC cells.
ZnO-NP inhibited proliferation, migration, and invasion and induced apoptosis of GC cells. Meanwhile, ZnO-NP significantly reduced the IC50 value of DDP for the inhibition of cell proliferation of DDP-resistant SGC7901/DDP cell lines. Autophagy was increased in the chemotherapy-resistant GC cells, as demonstrated by elevated LC3II/LC3I and Beclin-1 expression and repressed p62 expression in the SGC7901/ DDP compared with that in SGC7901 cells. Mechanically, ZnO-NP inhibited autophagy in GC cells, and treatment with DDP induced autophagy in the cells, which was reversed by ZnO-NP. Functionally, ZnO-NP attenuated the tumor growth of chemoresistant GC cells in vivo.
ZnO-NP alleviates the chemoresistance of GC cells by inhibiting autophagy. Our findings provide innovative insights into the scenario in which ZnO-NP mediates chemotherapy resistance in GC.
ZnO-NP may serve as a potential therapeutic candidate for GC treatment. The potential role of ZnO-NP in the clinical treatment of GC needs to be clarified in future investigations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farag N, Raiter A S-Editor: Fan JR L-Editor: A Filipodia P-Editor: Liu JH
| 1. | Marin JJ, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales JM, Macias RI. Mechanisms of Resistance to Chemotherapy in Gastric Cancer. Anticancer Agents Med Chem. 2016;16:318-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Ham IH, Oh HJ, Jin H, Bae CA, Jeon SM, Choi KS, Son SY, Han SU, Brekken RA, Lee D, Hur H. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019;18:68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 413] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 5. | Guo W, Deng L, Chen Z, Yu J, Liu H, Li T, Lin T, Chen H, Zhao M, Zhang L, Li G, Hu Y. Vitamin B12-conjugated sericin micelles for targeting CD320-overexpressed gastric cancer and reversing drug resistance. Nanomedicine (Lond). 2019;14:353-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Xu Z, Chen L, Xiao Z, Zhu Y, Jiang H, Jin Y, Gu C, Wu Y, Wang L, Zhang W, Zuo J, Zhou D, Luan J, Shen J. Potentiation of the anticancer effect of doxorubicinin drug-resistant gastric cancer cells by tanshinone IIA. Phytomedicine. 2018;51:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, Lee KH, Kim TY, Oh DY, Bang YJ, Im SA. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 9. | Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2692] [Cited by in F6Publishing: 2884] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 10. | Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2401] [Cited by in F6Publishing: 2580] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 11. | Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 1392] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 12. | Zhao F, Huang W, Zhang Z, Mao L, Han Y, Yan J, Lei M. Triptolide induces protective autophagy through activation of the CaMKKβ-AMPK signaling pathway in prostate cancer cells. Oncotarget. 2016;7:5366-5382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Xi Z, Si J, Nan J. LncRNA MALAT1 potentiates autophagyassociated cisplatin resistance by regulating the microRNA30b/autophagyrelated gene 5 axis in gastric cancer. Int J Oncol. 2019;54:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Hu YR, Yu YC, You SW, Li KQ, Tong XC, Chen SR, Chen ED, Lin XZ, Chen YF. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 15. | Caruso RA, Angelico G, Irato E, de Sarro R, Tuccari G, Ieni A. Autophagy in advanced low- and high-grade tubular adenocarcinomas of the stomach: An ultrastructural investigation. Ultrastruct Pathol. 2018;42:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Kim JS, Bae GE, Kim KH, Lee SI, Chung C, Lee D, Lee TH, Kwon IS, Yeo MK. Prognostic Significance of LC3B and p62/SQSTM1 Expression in Gastric Adenocarcinoma. Anticancer Res. 2019;39:6711-6722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Ieni A, Cardia R, Giuffrè G, Rigoli L, Caruso RA, Tuccari G. Immunohistochemical Expression of Autophagy-Related Proteins in Advanced Tubular Gastric Adenocarcinomas and Its Implications. Cancers (Basel). 2019;11:389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Cardoso VF, Francesko A, Ribeiro C, Bañobre-López M, Martins P, Lanceros-Mendez S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv Healthc Mater. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 19. | Hoang Thi TT, Cao VD, Nguyen TNQ, Hoang DT, Ngo VC, Nguyen DH. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;99:631-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Kim D, Shin K, Kwon SG, Hyeon T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv Mater. 2018;30:e1802309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11:313-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 651] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 22. | Zhang H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv Mater. 2020;32:e1806328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 23. | Chen G, Wang Y, Xie R, Gong S. A review on core-shell structured unimolecular nanoparticles for biomedical applications. Adv Drug Deliv Rev. 2018;130:58-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Tang KS. The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci. 2019;239:117011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Hu H, Guo Q, Fan X, Wei X, Yang D, Zhang B, Liu J, Wu Q, Oh Y, Feng Y, Chen K, Hou L, Gu N. Molecular mechanisms underlying zinc oxide nanoparticle induced insulin resistance in mice. Nanotoxicology. 2020;14:59-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Yadav KK, Arakha M, Das B, Mallick B, Jha S. Preferential binding to zinc oxide nanoparticle interface inhibits lysozyme fibrillation and cytotoxicity. Int J Biol Macromol. 2018;116:955-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Sun Q, Li J, Le T. Zinc Oxide Nanoparticle as a Novel Class of Antifungal Agents: Current Advances and Future Perspectives. J Agric Food Chem. 2018;66:11209-11220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Padmanabhan A, Kaushik M, Niranjan R, Richards JS, Ebright B, Venkatasubbu GD. Zinc Oxide nanoparticles induce oxidative and proteotoxic stress in ovarian cancer cells and trigger apoptosis Independent of p53-mutation status. Appl Surf Sci. 2019;487:807-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Li Z, Guo D, Yin X, Ding S, Shen M, Zhang R, Wang Y, Xu R. Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/Caspase-9/Caspase-3 signaling pathway in vitro. Biomed Pharmacother. 2020;122:109712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Bai DP, Zhang XF, Zhang GL, Huang YF, Gurunathan S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int J Nanomedicine. 2017;12:6521-6535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Ruenraroengsak P, Kiryushko D, Theodorou IG, Klosowski MM, Taylor ER, Niriella T, Palmieri C, Yagüe E, Ryan MP, Coombes RC, Xie F, Porter AE. Frizzled-7-targeted delivery of zinc oxide nanoparticles to drug-resistant breast cancer cells. Nanoscale. 2019;11:12858-12870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Sun Z, Song X, Li X, Su T, Qi S, Qiao R, Wang F, Huan Y, Yang W, Wang J, Nie Y, Wu K, Gao M, Cao F. In vivo multimodality imaging of miRNA-16 iron nanoparticle reversing drug resistance to chemotherapy in a mouse gastric cancer model. Nanoscale. 2014;6:14343-14353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Li LQ, Pan D, Zhang SW, -Y-Xie D, Zheng XL, Chen H. Autophagy regulates chemoresistance of gastric cancer stem cells via the Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:3402-3407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 34. | Xiao F, Ouyang B, Zou J, Yang Y, Yi L, Yan H. Trim14 promotes autophagy and chemotherapy resistance of gastric cancer cells by regulating AMPK/mTOR pathway. Drug Dev Res. 2020;81:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Lu R, Zhao G, Yang Y, Jiang Z, Cai J, Hu H. Inhibition of CD133 Overcomes Cisplatin Resistance Through Inhibiting PI3K/AKT/mTOR Signaling Pathway and Autophagy in CD133-Positive Gastric Cancer Cells. Technol Cancer Res Treat. 2019;18:1533033819864311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Sun Y, Jiang Y, Huang J, Chen H, Liao Y, Yang Z. CISD2 enhances the chemosensitivity of gastric cancer through the enhancement of 5-FU-induced apoptosis and the inhibition of autophagy by AKT/mTOR pathway. Cancer Med. 2017;6:2331-2346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |