Published online Jan 7, 2021. doi: 10.3748/wjg.v27.i1.1
Peer-review started: September 3, 2020
First decision: October 17, 2020
Revised: November 1, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: January 7, 2021
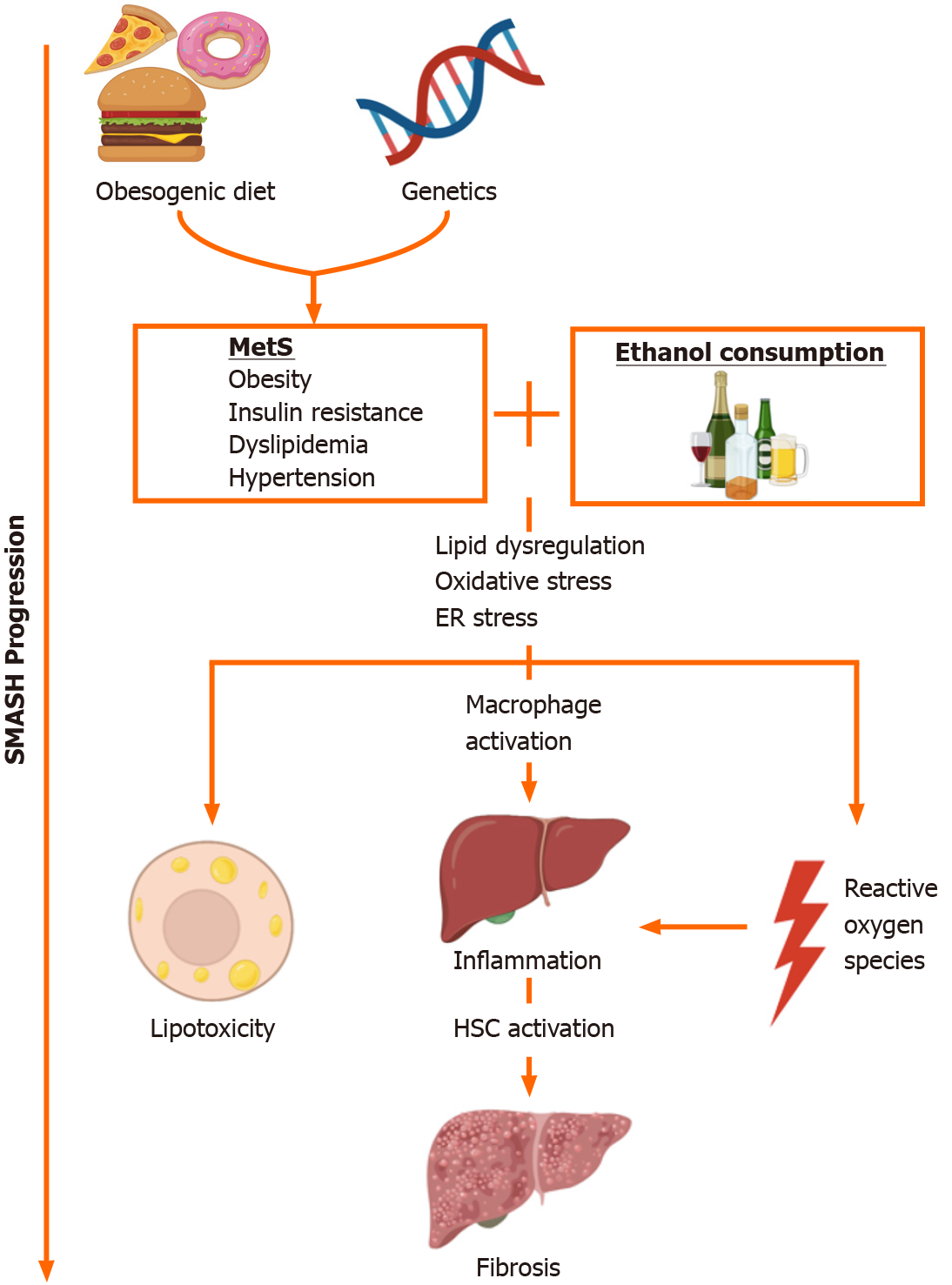
Non-alcoholic fatty liver disease (NAFLD) is a multi-systemic disease that is considered the hepatic manifestation of metabolic syndrome (MetS). Because alcohol consumption in NAFLD patients is common, there is a significant overlap in the pathogenesis of NAFLD and alcoholic liver disease (ALD). Indeed, MetS also significantly contributes to liver injury in ALD patients. This “syndrome of metabolic and alcoholic steatohepatitis” (SMASH) is thus expected to be a more prevalent presentation in liver patients, as the obesity epidemic continues. Several pre-clinical experimental models that couple alcohol consumption with NAFLD-inducing diet or genetic obesity have been developed to better understand the pathogenic mechanisms of SMASH. These models indicate that concomitant MetS and alcohol contribute to lipid dysregulation, oxidative stress, and the induction of innate immune response. There are significant limitations in the applicability of these models to human disease, such as the ability to induce advanced liver injury or replicate patterns in human food/alcohol consumption. Thus, there remains a need to develop models that accurately replicate patterns of obesogenic diet and alcohol consumption in SMASH patients.
Core Tip: Experimental animal and cell culture models have been developed to study the “syndrome of metabolic and alcoholic steatohepatitis” (SMASH), in which concomitant non-alcoholic fatty liver disease and alcoholic liver disease risk factors play a role in liver injury. These models demonstrate that obesity, metabolic syndrome, and alcohol consumption synergistically contribute to lipid dysregulation, oxidative stress, inflammation, and fibrogenesis. The pathogenesis of SMASH in these experimental models is dependent on obesogenic diet composition, alcohol consumption patterns, alcohol dosage, and genetic background.
- Citation: Buyco DG, Martin J, Jeon S, Hooks R, Lin C, Carr R. Experimental models of metabolic and alcoholic fatty liver disease. World J Gastroenterol 2021; 27(1): 1-18
- URL: https://www.wjgnet.com/1007-9327/full/v27/i1/1.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i1.1
The two most common causes of liver disease worldwide are non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD)[1]. NAFLD is a multi-system disease whose major risk factors are genetic susceptibility, obesity, insulin resistance, and metabolic syndrome (MetS). MetS is the constellation of obesity, insulin resistance, hypercholesterolemia, hypertriglyceridemia, and hypertension; and NAFLD is considered the hepatic manifestation of MetS[2-4]. ALD also has a multi-factorial etiology, including alcohol consumption quantity and pattern, environmental factors, and genetics. Alcohol consumption patterns can be described in terms of long-term (chronic) or acute (binge) drinking episodes.
Clinicians recognize the existence of an overlap condition between NAFLD and ALD, despite the absence of significant alcohol intake in NAFLD diagnostic criteria[5]. For example, among ALD patients, obesity increases the likelihood for the development of alcoholic cirrhosis[6], while both long-term alcohol consumption and obesity have been found to independently promote advanced fibrosis[7]. Obesity and MetS also increase mortality in ALD[8]. Obesity, insulin resistance, and MetS are the most significant contributors to ALD severity, with as many as 50% of ALD patients estimated to have liver disease as a consequence of both alcohol overconsumption and obesity[9,10]. Obese individuals who overconsume alcohol are more likely to develop hepatic steatosis and cirrhosis compared to normal weight individuals[7]. As the obesity epidemic persists, this “syndrome of metabolic and alcoholic steatohepatitis” or SMASH is expected to become a more common presentation in liver patients[11].
Several experimental models have been developed to understand the metabolic factors that exacerbate liver disease in patients with coexistent NAFLD and ALD risk factors. Studies that incorporated both obesogenic diet (e.g., high-fat, high-fructose) or genetic obesity and some form of alcohol consumption in a rodent model were included in this critical review, while human retrospective studies, human clinical trials, and in vitro models were excluded. While these models couple NAFLD risk factors with alcohol exposure, they differ in the animal genetic background, obesogenic diet composition, type and duration of alcohol consumption, and ability to induce significant liver injury. Here, we review the pre-clinical experimental models developed to study the interactive effects of NAFLD and ALD risk factors on hepatic injury, as well as limitations of the various models with regards to human disease.
Lipid metabolism dysregulation is a key factor in the pathogenesis of NAFLD and ALD. De novo lipogenesis and fatty acid oxidation (FAO) are implicated in NAFLD pathogenesis, although lipid uptake, storage, and export also play a role[12]. Meanwhile, experimental models indicate that alcohol (EtOH) consumption further exacerbates lipid dysregulation in MetS. AMP-activated protein kinase (AMPK) is a key regulator of lipid metabolism, and its inhibition has been implicated in both NAFLD and ALD[13-15]. AMPK has reciprocal effects on de novo lipogenesis and fatty acid (FA) uptake through the regulation of the transcription factor sterol response element binding protein 1 (SREBP-1)[16]. SREBP-1 is upregulated in NAFLD patients and in high-fat diet (HFD)-induced NAFLD rodent models[17]. Pharmacological inhibition of SREBP-1 increases insulin sensitivity and suppresses FA synthesis[18]. AMPK also modulates FAO by regulating peroxisome proliferator-activated receptor (PPAR) α and sirtuin 1 (SIRT-1), the latter of which is inhibited by EtOH con-sumption[19-22]. FAO is induced in NAFLD as a compensatory effect of increased lipid uptake and de novo lipogenesis[23]. In obese patients, EtOH is also known to increase adiponectin levels, which induces AMPK activation[24].
NAFLD risk factors and EtOH consumption also contribute to oxidative stress in fatty liver disease by dysregulating oxidative biochemical processes and producing reactive oxygen species (ROS). Obesogenic diet contributes to the formation of ROS such as hydrogen peroxide (H2O2), superoxide (O2-), 4-hydroxynonenal (4-HNE), malondialdehyde (MDA), and oxysterols as byproducts of FAO[25]. EtOH metabolism by alcohol and acetaldehyde dehydrogenases, and cytochrome P450 2E1 (CYP2E1) also produces ROS[26]. CYP2E1, in particular, is implicated in the pathogenesis of both NAFLD and ALD in humans[5,27]. CYP2E1 metabolizes EtOH and other xenobiotics in a nicotinamide adenine dinucleotide phosphate (NADP+/NADPH)-mediated process and produces H2O2 and O2–[28]. Mouse models also indicate that CYP2E1 plays a role in lipid dysregulation[29] and diabetes[30]. Inhibition of CYP2E1 has been found to be protective against lipid metabolism dysregulation and oxidative stress induced by combined FA and EtOH treatment in vitro[31].
Concomitant NAFLD risk factors and EtOH consumption also dysregulate the unfolded protein response (UPR), resulting in the production of ROS in the endoplasmic reticulum (ER)[32]. In the UPR, misfolded proteins sequester binding immunoglobulin protein (BIP), inducing the protein kinase R-like ER kinase (PERK), activating transcription factor 6 (ATF-6), and inositol-requiring kinase (IRE-1) transduction pathways. The UPR involves the formation of disulfide bonds, an oxidative process that produces H2O2 and other ROS[32]. Protein folding dysregulation is associated with oxidative stress in fatty liver disease[33]; saturated FA, oleic acid, and cholesterol interact with the UPR and induce ER stress[34]. Lipids and EtOH have a reciprocal relationship with the ER: PERK induces protein and lipid metabolism, and apoptosis, while ATF-6 and IRE-1 mediate protein trafficking and inflammation[35,36].
Three enzymes are key to the elimination of ROS: Superoxide dismutase (SOD) metabolizes O2- to O2 and H2O2; while catalase and glutathione peroxidase (GPx) metabolize H2O2 to O2 and H2O[37,38]. H2O2 oxidation by GPx is coupled to the metabolism of reduced glutathione (GSH) to oxidized glutathione disulfide (GSSG). GSSG is reduced back to GSH by glutathione reductase (GR), which requires NADPH[39,40]. Clinical observations indicate that patients with NAFLD have increased SOD, GPx, and GR activities, as well as elevated levels of GSH[41,42]. Similarly, patients with ALD have upregulated SOD activity[43], although GSH/GSSG ratio is decreased[44].
Macrophage and neutrophil activation play important roles in liver injury in the context of MetS and EtOH consumption[45,46]. Macrophages adopt a number of functional states that are traditionally categorized into two groups. Pro-inflammatory M1 polarization is distinguished by the presence of cell surface marker CD68, and secretion of interleukin (IL) 6, tumor necrosis factor α (TNFα), and other cytokines. Conversely, anti-inflammatory M2 polarization is distinguished by CD163, transforming growth factor β (TGFβ), IL-10, and arginase 1 (ARG-1)[47]. Saturated FAs and cholesterol are known to induce a predominantly M1-like phenotype in Kupffer cells, liver-resident macrophages[48]. M1 polarization is associated with severity of disease, CD68+ Kupffer cells, and TNFα expression were both found to be significantly higher in non-alcoholic steatohepatitis (NASH) patients compared to patients with simple steatosis[49]. However, CD45+ leukocytes and CD163+ Kupffer cells are also increased in pediatric NAFLD patients[50]. Meanwhile, hepatocyte damage in ALD increases lipopolysaccharide (LPS) leakage into the liver by enhancing intestinal permeability, resulting in TNFα and IL-10 secretion by Kupffer cells[51,52]. The two-state model of macrophage polarization may thus be insufficient to describe immune response under concomitant NAFLD and ALD risk factors. Kupffer cells also release IL-1β in response to hepatic tissue damage and necrosis, which results in neutrophil recruitment from bone marrow to the liver[53]. Neutrophil infiltration, distinguished by upregulation in TNFα, IL-1, and osteopontin (OPN), contributes to hepatocyte death and correlates with ALD severity[54].
NAFLD pathogenesis involves hepatocellular apoptosis, which occurs via the extrinsic and intrinsic pathways. In the intrinsic pathway, apoptosis occurs as a result of cellular, ER, or mitochondrial stress[55]. In the extrinsic pathway, apoptosis arises as a response to extracellular signaling[56]. One particularly important extrinsic mechanism in NAFLD is the tumor necrosis factor α (TNFα) pathway. TNFα activates anti-apoptotic NF-κB and apoptotic caspase-3[57,58].
Oxidative stress, inflammation, and hepatocyte apoptosis contribute to hepatic fibrosis in NAFLD and ALD, and these pathways are mediated by hepatic stellate cells (HSC)[59]. One important fibrogenic mechanism in NAFLD and ALD is the TGFβ pathway, which induces the expression of collagens, a component of extracellular matrix (ECM), via the signal transducer SMAD-3. Another important signaling pathway is that of toll-like receptor 4 (TLR-4), which responds to LPS, and produces pro-inflammatory cytokines[60,61]. TLR-4 is upregulated in obese patients with NAFLD[62]; and in patients with ALD, alcohol increases gut permeability and promotes LPS-producing Gram-negative bacteria[63]. However, in one study, mRNA expression of TLR-4 tended to decrease in biopsy-diagnosed NAFLD patients who drank at most 20 g/kg/d EtOH compared to those who did not drink[64]. Activated HSCs also produce tissue inhibitor of metalloproteinases (TIMP), which inhibits ECM-degrading matrix metalloproteinases (MMPs)[65].
Despite the pathological overlap between NAFLD and ALD, most studies model NAFLD risk factors and EtOH consumption independent of each other. Although individually these models are limited in their capacity to recapitulate SMASH pathogenesis, they form the foundation for many SMASH models.
A variety of animal models have been proposed for studying NAFLD, which can be largely divided into three categories: dietary, genetic, and combined models. Dietary manipulations can induce NAFLD development by using obesogenic or nutrient-deficient diets[66]. Obesogenic diets are typically rich in fat with additional sugar and/or cholesterol, representing metabolic and histological features of human NAFLD. There are a number of obesogenic diets, varying in the macronutrient composition, sources of fat, types of sugar, and cholesterol content. Nutrient-deficient diets, such as methionine- and choline-deficient, and choline-deficient L-amino acid-defined (CDAA) diets, are also capable of inducing NAFLD[66-68]. Choline-deficient and CDAA diets are used because methionine and choline are required for phosphatidylcholine synthesis, and are thus intimately involved in lipoprotein synthesis, lipid storage, and lipid regulation[69]. Nutrient-deficient diets can rapidly induce NAFLD within a few weeks of feeding, but some metabolic features including body weight, hyperglycemia, and insulin sensitivity do not manifest as they do in human NAFLD.
Various genetic animal models have also been created to study NAFLD, and are especially valuable for studying the mechanisms of NAFLD pathogenesis[67,70]. Genetic mouse models of NAFLD include leptin-deficient (ob/ob), leptin receptor-deficient (db/db), insulin resistant (KK-Ay), SREBP-1c transgenic, PPARα null, phosphatase and tensin homolog (PTEN) null, and acyl-coenzyme A oxidase (ACOX) null mice. For rats, genetic models include leptin receptor-deficient (fa/fa) and cholecystokinin knockout (OLETF). Dietary and genetic models are also combined to replicate the multifactorial nature of human NAFLD. A detailed discussion of NAFLD animal models is beyond the scope of this review and described in other reviews[66-68,70].
Pre-clinical models have also been developed to understand the pathogenesis of ALD. EtOH can be administered to animals either orally or intravenously. Four models have been developed for studying ALD: (1) Binge feeding, in which animals are acutely administered EtOH by gavage; (2) Chronic feeding, in which animals are given access to EtOH through diet or drinking water for a prolonged period of time (e.g., Lieber-DeCarli diet model[71]); (3) Intragastric (iG) infusion feeding (e.g., Tsukamoto-French model[72-74]); and (4) Chronic-binge feeding, in which animals are given chronic EtOH for a certain period of time, then administered EtOH acutely (e.g., Gao model[75,76]). Variations in these models exist, such as multiple binge feeding and combined models. In addition, experimental models vary in EtOH concentration and feeding period. Animal models should thus be selected depending on the purpose of the study, as there have been no animal models that fully recapitulate the full spectrum of ALD.
Experimental rodent models (Table 1) of SMASH couple one of the dietary or genetic models of NAFLD described above with EtOH consumption models of ALD. While obesity and EtOH consumption models vary considerably, studies that combine the two generally indicate a synergistic increase in steatosis, inflammation, and fibrosis by obesity and EtOH.
| Ref. | Experimental model details | Biochemistry | Anatomy and histology | mRNA and protein expression | |
| Alwahsh et al[84] | (1) Animal: 10 wk male Sprague- Dawley rat; (2) Initial weight: 270-310 g; (3) Diet: LD control, LD with 30% kcal from fructose, 30% kcal from EtOH, or LD with both fructose and EtOH; and (4) Duration: 28 d | (1) ↑ ALT; (2) ↑ liver and plasma TG; (3) ↑ plasma leptin; (4) ↓ plasma HDL; (5) ↓ plasma albumin; and (6) No dif. in leptin or hepatic TG between LD-EtOH and LD- fructose-EtOH | (1) Portal inflammatory infiltration and stage 1 fibrosis (LD-EtOH); (2) Periportal macrosteatosis (LD- fructose); and (3) Portal inflammation, periportal macrosteatosis, fibrosis (LD- fructose-EtOH) | (1) ↑ leptin; (2) ↑ ACC-2; (3) ↑ lipase in LD-EtOH, but ↓ in LD-fructose and LD-fructose-EtOH; (4) ↓ IRS-1, IRS-2 (fructose groups); and (5) ↑ CD36, CPT-1α, PPARα in LD- EtOH and LD-fructose, but no change in LD-fructose-EtOH | |
| Bucher et al[112] | (1) Animal: 7 wk male C57BL/6J Mouse; (2) Initial weight: 20-23 g; (3) Diet: Control (3 kcal/g food; 16% kcal protein) or HFD (5.5 kcal/g; 60% kcal SFA); 10 g/kg/d EtOH in drinking water; and (4) Duration: 4 mo | (1) ↑ ALT, but ↓ ALT compared to EtOH-naïve HFD; (2) ↑ cholesterol, but ↓ cholesterol compared to EtOH-naïve HFD; (3) ↑ TG↑ serum glucose; (4) ↑ serum insulin; and (5) ↑ MUFA and ↓ SFA (compared to HFD) | (1) Mediovesicular and macrovacular steatosis and (2) No dif. in necroinflammation or perisinusoidal fibrosis between HFD and HFD-EtOH | (1) ↑ genes for apoptosis inhibition, acetyl-CoA synthesis, lipogenesis, mitochondrial functions (NADH dehydrogenase, COX, ATP synthase), and proteolysis; (2) ↓ genes for apoptosis (BCL-2 homologs), fibrosis (collagen), chemotaxis, oxidative stress (GPx, HMOX-1, SOD); and (3) ↓ CYP2E1 protein levels | |
| Carmiel-Haggai et al[94] | (1) Animal: 15 wk male fa/fa Zucker Rat; (2) Initial weight: 595 ± 35 g (obese), 316 ± 32 g (lean controls); and (3) Diet: 35% v/v EtOH in saline every 12 h for 3 d; final dose was 4 g/kg EtOH | (1) ↑ ALT; (2) ↑ NEFA; (3) ↑ LPO by-products (4-HNE, MDA); and (4) ↓ CYP2E1 activity | (1) Macrovesicular steatosis (EtOH- naïve fa/fa) and (2) Lobular microvesicular, central macrosteatosis, inflammation (EtOH-fed fa/fa) | (1) ↓ GSH, GPx, GR; (2) ↓ GSSG (EtOH-fed and EtOH-naïve fa/fa); (3) ↓ catalase, SOD; (4) ↑ iNOS; (5) ↑ caspase 3, caspase 8; (6) ↑ BCL-XL, FAS ligand; and (7) ↑ BCL-2, BAX (EtOH-fed and EtOH-naïve fa/fa) | |
| Duly et al[80] | (1) Animal: 6-8 wk male C57BL/6 Mouse; (2) Initial weight: 20 g; (3) Diet: chow (12% kcal fat) or HFD (45% kcal fat, 0.25% cholesterol); 2 g/kg EtOH in saline twice per wk; and (4) Duration: 12 wk | (1) ↑ TG (chow-EtOH, but not HFD or HFD-EtOH); (2) ↑ cholesterol, HDL, LDL (but no difference between HFD and HFD- EtOH); and (3) ↑ serum insulin | (1) Steatosis and lipid accumulation; (2) Collagen deposition; (3) ↑ cellular infiltration; (4) ↑ CD45+ leukocytes; (5) ↑ F4/80+ Kupffer cells; and (6) ↑ vimentin+ HSCs | (1) ↑ SREBP-1; (2) ↑ SCD-1; (3) ↑ PPARα; (4) ↓ ACOX-1; (5) No dif. in TGFβ or HSP90; and (6) ↑ collagen I, PAI-1 (EtOH-naïve HFD) | |
| Everitt et al[87] | (1) Animal: 12 wk male ob/ob C57BL/6-J/Rj-ob mouse; (2) Diet: PUFA-enriched LD with 27.5% EtOH or isocaloric maltodextrin; and (3) Duration: 4 wk | (1) ↑ ALT/AST; (2) ↑ hepatic TG; (3) ↑ hepatic cholesterol; (4) ↑ hepatic lactate; (5) ↓ hepatic pyruvate; (6) No dif. in BAL between EtOH-fed; and (7) ob/ob and EtOH-fed lean mice | Steatosis in EtOH-fed ob/ob | (1) ↑ mTOR, PPARγ, FGF-21, FSP-27; (2) ↑ SIRT-1, pAMPKα, AMPKα, pACC (ob compared to lean; EtOH did not have an effect); (3) ↑ adipose TNFα, ↓ hepatic TNFα; (4) ↑ cytosolic lipin-1 protein levels; (5) ↓ nuclear lipin-1 protein levels; (6) ↓ PGC-1α; and (7) ↓ ACOX-1 | |
| Gäbele et al[77] | (1) Animal: 12 wk female Balb/c mouse; (2) Diet: Chow or HFD [17% fat (50% lard, 50% cacao-butter), 1.25% cholesterol, 0.5% cholate]; 5% EtOH in ad libitum; and (3) Duration: 6 wk | (1) ↑ hepatic TG; (2) ↑ portal blood LPS; and (3) ↓ BAL (not statistically significant) | (1) Steatosis in HFD-fed mice; (2) ↑ 4-HNE; (3) ↑ αSMA+ cells; (4) ↑ collagen I; and (5) ↑ ECM deposition | (1) ↑ p47phox, TNF, TGFβ, collagen I; (2) ↑ TLR-4 in HFD mice, with no effect by EtOH; (3) ↑ αSMA protein levels; and (4) ↓ CYP2E1 protein levels in HFD | |
| Gopal et al[79] | (1) Animal: 6-8 wk male C57BL/6 mouse; (2) Diet: Chow or HFD (45% kcal fat) for 10 wk, then LD control or 5% v/v EtOH LD for 4 wk additionally; and (3) Drug: HFD mice also given 1000 U/kg Cu/Zn SOD-1 with polylysine- PEG copolymer in 10 mM HEPES every 2 d for 2 wk | (1) ↑ ALT; (2) ↑ FFA; (3) ↓ leptin; (4) nanoSOD treatment counteracted the above effects; and (5) No dif. in MDA between groups | (1) ↑ adipose mass by HFD, but ↓ fat/body weight ratio by HFD- EtOH; (2) ↑ microvesicular steatosis (in all mice); (3) ↑ macrovesicular steatosis; (4) ↑ inflammatory nodules; (5) ↑ hepatocyte ballooning; and (6) ↑ MCP-1 protein levels, ↓ MCP-1 in HFD-EtOH mice treated with nanoSOD | (1) ↑ adipose CYP2E1 protein levels, ↓ CYP2E1 by nanoSOD; (2) ↑ hepatic CYP2E1, ADH, catalase, ↓ CYP2E1 and ADH, but ↑ catalase by nanoSOD; (3) ↑ PPARα, ACOX-1 in HFD, further ↑ ACOX-1 by EtOH, further ↑ for both by nanoSOD; and (4) ↑ CCL-2, MMP-12; ↓ by nanoSOD | |
| Jung et al[108] | (1) Animal: Female C57BL/6J mouse; (2) Diet: Control (69% carbohydrates, 12% fat, 19% protein) or FFC (60% carbohydrates, 25% fat, 15% protein, 50% wt/wt fructose, 0.16% wt/wt cholesterol); Diets enriched with 2.5 g/kg EtOH, isocaloric beer (4.9% v/v EtOH), or isocaloric diet; and (3) Duration: 7 wk | (1) ↑ TG; ↓ in beer-treated FFC compared to EtOH-treated or naïve and (2) ↓ glucose tolerance in all FFC-fed groups | (1) ↑ neutrophil granulocytes; ↓ by beer in FFC-fed mice; (2) ↑ NAS; ↓ by beer in FFC-fed mice; (3) ↑ iNOS protein levels; ↓ by beer and EtOH in FFC-fed mice; and (4) ↑ 4-HNE; ↓ by beer and EtOH in FFC-fed mice | (1) ↑ IR, IRS-2 in FFC-EtOH and FFC- beer compared to naïve; no dif. in IRS-1; (2) ↓ adiponectin in FFC and FFC- EtOH, but ↑ in FFC-beer; (3) ↑ hepatic AdipoR1 and SIRT-1 in FFC-beer; and (4) No dif. in PPARγ, AdipoR2, FASN, SREBP-1c, ACOX-1 between groups | |
| Kanuri et al[106] | (1) Animal: 6 wk male ob/ob C57BL/6 mouse; (2) Diet: 2.5 g/kg/d EtOH in drinking water; and (3) Duration: 6 wk | (1) ↑ ALT/AST in ob/ob, but ↓ slightly by EtOH in ob/ob; (2) ↑ TG, cholesterol, HDL, LDL in ob/ob, but no effect by EtOH; and (3) No dif. in FFA | (1) Steatosis, hepatomegaly in ob/ob; (2) Macrovesicular steatosis in EtOH- naïve ob/ob; (3) Microvesicular steatosis in EtOH- fed ob/ob; and (4) ↓ hepatic neutrophils | (1) ↑ PLIN-2, PLIN-3, TNFα, PAI-1 in ob/ob, ↓ PAI-1, PLIN-2, PLIN-3 by EtOH; (2) ↓ IRS-1, IRS-2, adiponectin, SIRT- 1 in ob/ob; ↑ adiponectin, SIRT-1 by EtOH; (3) No effect by EtOH on PPARγ, ACOX-1, SREBP-1c, IRS-1, IRS02, GLUT-4, glucokinase, PEPCK; and (4) No dif. in hepatic ACOX-1, PPARγ | |
| Kitagawa et al[93] | (1) Animal: 8 wk female KK-Ay mouse; (2) Diet: LD with 5% EtOH or isocaloric maltodextrin; Single gavage of 4 g/kg EtOH or gavage on after 11 d; (3) Drug: 0.1 g/L rifaximin (RFX); and (4) Duration: 10 d | (1) ↑ ALT; (2) ↑ serum and hepatic TG; (3) ↑ portal blood LPS; (4) ↑ CYP2E1 activity; and (5) ↓ ALT, TG, LPS (but not to basal levels) in EtOH-fed treated with RFX | (1) Lipid droplet accumulation in hepatic lobule; (2) Hepatomegaly; (3) ↑ 4-HNE; and (4) RFX counteractedthe aboveeffects | (1) ↑ ACCα, FASN; TNFα, IL-6, ILNγ; CCL-2; CD14, TLR-4, TLR-2; HMOX- 1, NOX-1; (2) ↓ CPT-1α; (3) ↑ CYP2E1 protein levels; no effect by RFX; and (4) RFX counteracted effects of EtOH on ACCα, FASN, TNFα, IFNγ, TLR-4, HMOX-1, and NOX-2, CPT-1α | |
| Lazaro et al[81] | (1) Animal: Male WT and OPN- knockout C57BL/6 mice and (2) Diet: High-fat, high-cholesterol diet for 2 wk, then iG liquid HFD-EtOH (36% kcal from corn oil; 27 g/kg/d EtOH) or isocaloric HFD for 8 wk; Some HFD-EtOH given 4-5 g/kg/wk EtOH by gavage | (1) ↑ ALT, but ↓ with binge EtOH; ↓ ALT in binge-treated OPN KO compared to binge-treated WT; (2) ↑ bilirubin, ↓ albumin; (3) ↑ TG; and (4) No dif. in BAL | (1) Steatosis, hepatomegaly, mononuclear cell inflammation, neutrophil infiltration, perisinusoidal and percellular fibrosis in HFD-EtOH and (2) Splenomegaly, ↑ TLR-4 in binge-treated mice; effect of binge greater in OPN KO | (1) ↑ collagen I, αSMA, TIMP-1 by EtOH; further ↑ by EtOH binge; (2) ↑ myeloperoxidase, CXCL-1, OPN in binge-treated; and (3) ↑ myeloperoxidase, IL-17α in binge-treated OPN KO | |
| Minato et al[96] | (1) Animal: 30 wk male OLET and OLETF rat; (2) Initial weight: 620 ± 15 g (OLETF), 460 ± 10 g (OLET); (3) Diet: 10 mL 10% EtOH by gavage for 1-5 d/wk; and (4) Duration: 3 wk | (1) ↑ ALT/AST; (2) ↑ serum and hepatic TG; (3) ↑ serum glucose; (4) ↑ serum insulin in OLETF, with no effect from EtOH; and (5) ↑ serum adiponectin in OLETF, ↓ by EtOH in OLETF | (1) Mild pericentral microvesicular steatosis in EtOH-naïve OLETF; (2) Steatohepatitis, focal hepatic necrosis, and hepatocyte ballooning in EtOH-treated OLETF; (3) ↑ hepatic CYP2E1; and (4) ↑ 4-ΗΝΕ | (1) ↑ PPARγ in OLETF, with no effect from EtOH and (2) ↑ TNFα | |
| Nieto et al[85] | (1) Animal: 12 wk male Lewis rat; (2) Initial weight: 100 g; (3) Diet: Choline deficient (CD) diet; (4) 15 mL/kg whiskey by gavage 3 times per wk; and (5) Duration: 3 mo | (1) ↑ ALT/AST; (2) ↑ TG; (3) ↑ NEFA; (4) ↑ MDA, protein carbonyls; (5) ↑ serum TNFα, but ↓ in CD- whiskey; (6) ↑ CYP2E1 activity; (7) ↑ caspase-3, caspase-8 activity | (1) Minimal steatosis in whiskey-naïve CD rats and (2) Periportal and percentral microvesicular steatosis in CD-whiskey rats | (1) ↑ collagen 1, αSMA, cytochrome c, HSP47, CYP2E1; (2) ↑ leptin, TIMP-1, and BAX protein levels by CD, with no effect by whiskey; and (3) ↓ MMP-2, MMP-9, MMP-13, BCL-2, BCL-XL by CD, with no effect by whiskey | |
| Osaki et al[101] | (1) Animal: Male Sprague-Dawley rat; (2) Initial weight: 106 g; (3) Diet: HFD (30% beef tallow, 20% casein, 20% sucrose); 1%-2% v/v EtOH ad libitum; and (4) Duration: 12 wk | (1) ↓ ALT; no dif. in AST; (2) ↓ ammonia, urate; (3) No dif. in serum glucose, FFA, cholesterol, HDL, bilirubin, alkaline phosphatase levels, urea, creatinine, lactate, acetate; (4) No dif. in hepatic TG, MDA; (5) No dif. in hepatic, renal, or pulmonary thiobarbituric acid-reactive substances | No dif. in hepatic TG, MDA No dif. in weight of liver, epididymal adipose tissue, perirenal adipose tissue, or gastrocnemius muscle | No dif. in TNFα, adiponectin, insulin-like growth factor binding protein 1, hemoglobin | |
| Robin et al[86] | (1) Animal: Male ob/ob C57BL/6J mouse; (2) Initial weight: 49-56 g (obese), 26- 30 g (lean); (3) Diet: 2.5 g/kg/d EtOH by gastric intubation; (4) Drug: 100 mg/kg/d pentoxifylline (PTX) by iP; and (5) Duration: 4 d | (1) ↑ ALT in ob/ob; no effect by EtOH; (2) ↑ caspase-3,CYP2E1, SOD activities; ↓ caspase 3 by PTX; (3) ↓ GPx, cytosolic GT activity in ob/ob, but no effect by EtOH; (4) ↓ mitochondrial GT activity by EtOH; and (5) No dif. in GR activity | (1) Steatosis, caspase-3 activation in ob/ob; (2) TUNEL+ hepatocytes; and (3) Necrosis, inflammation in EtOH-naïve ob/ob | (1) ↑ TNFα protein levels; ↓ by PTX; (2) ↑ IκBα protein levels, ↓ NF-κB p65; ↓ NF-κB activity; (3) ↓ iNOS, BCL-XL; (4) ↓ cytosolic GSH, mitochondrial GSSG; and (5) ↑ HSP70 | |
| Song et al[124] | (1) Animal: 6 wk male C57BL/6J mouse; (2) Diet: high-fructose diet (20.2% kcal protein, 66.96% kcal carbohydrates, 12.9% kcal fat); 10% v/v EtOH for 2 d, 15% for 5 d, 20% for 17 wk; and (3) Duration: 18 wk | (1) ↑ ALT/AST; (2) ↑ plasma TG, NEFA, cholesterol by EtOH, but not fructose; (3) ↑ plasma glucose by fructose diet, ↓ glucose by EtOH; (4) ↑ insulin by fructose and EtOH, but not combined fructose-EtOH; (5) ↓ copper by fructose and EtOH, but not combined fructose-EtOH | (1) ↑ microvesicular steatosis and (2) ↑ macrovesicular steatosis in EtOH-naïve fructose; ↓ by fructose-EtOH diet | (1) ↑ hepatic CYP2E1 protein levels; (2) ↑ KHK in EtOH-naïve fructose; (3) ↑ F4/80, CD68, and TNFα, MyD88, IRF-3, and TRAF-6; (4) ↑ CCL-2, TLR-4, and IRF-7 only in fructose-EtOH; (5) ↑ CD163, IL-1β, or ARG-1 by EtOH, but no effect by fructose; and (6) No dif. in IL-6, IL-10 | |
| Sun et al[107] | (1) Animal: Male Sprague-Dawley rats; (2) Initial weight: 80-100 g; (3) Diet: HFD (38% fat, 2% cholesterol, 1% cholate) or control (38% kcal from fat); 4 g/kg/d EtOH by gavage beginning after 3 wk; and (4) Duration: 12 wk | No dif. in plasma LPS levels | (1) ↑ steatosis, fibrosis in HFD mice compared to HFD-EtOH and (2) ↑ Kupffer cell and HSC activation in HFD mice compared to HFD- EtOH | ↑ inflammatory cytokines (TNFα, CXCL-1, CXCL-2, IL-1β, IL-6), pro- fibrotic markers (αSMA, collagen, TGF-β, TIMP-1, MMP-2, MMP-9) in HFD compared to HFD-EtOH | |
| Suzuki et al[92] | (1) Animal: 8 wk male KK-Ay mouse; (2) Diet: LD containing 5% EtOH or isocaloric maltodextrin; 4 g/kg EtOH or isocaloric maltodextrin by gavage on day 11; (3) Drug: 120 mg/kg/d phenylbutyric acid (PBA); and (4) Duration: 10 d | (1) ↑ ALT/AST in chronic-binge mice; no dif. in ALT/AST between chronic-only and controls; (2) ↑ serum and hepatic TG; (3) No dif. in blood glucose; and (4) PBA ↓ ALT/AST, TG in EtOH-fed mice compared to PBA-naïve | (1) ↑ steatosis, PMN infiltration, ccCK18+ hepatocytes, 4-HNE+ cells in chronic-binge mice and (2) PBA alleviated liver injury, but did not decrease 4-HNE+ cell count | (1) ↑ inflammatory cytokines (TNFα, IL-6), ER stress markers (BiP, uXBP-1, sXBP-1, IP3R1, CHOP), and HMOX-1 by chronic-binge; PBA ↓ aforementioned genes; (2) ↑ BiP, uXBP-1 in chronic, but not sXBP-1, IP3R1, CHOP, HMOX-1; and (3) ↑ CYP2E1; PBA had no effect | |
| Wang et al[78] | (1) Animal: 8 wk male Sprague-Dawley rat; (2) Diet: LD control (35% kcal from fat) or LD HFD (71% kcal from fat) for 6 wk; then LD HFD were given either solid HFD (55% kcal fat) or HF-EtOH diet (55% kcal fat, 16% kcal EtOH) for 4 wk; (3) Duration: 10 wk | (1) ↑ TUNEL+ hepatocytes; (2) ↑ cleaved caspase-3 levels; and (3) No dif. in 4-HNE or MDA levels between HFD and HFD-EtOH, but ↑ compared to controls | (1) Lipid droplet accumulation; (2) ↑ steatosis; and (3) ↑ number of inflammatory foci | (1) ↑ Fas death receptor and ligand; (2) No dif. in caspase-3 expression; (3) No dif. in CYP2E1, TNFα, TNFR-1, IL-1β, IL-12 between HFD and HFD-EtOH, but ↑ compared to controls | |
| Xu et al[83] | (1) Animal: 8 wk male C57BL/6J mouse; (2) Diet: 580 kcal/kg/d (control) or 986 kcal/kg/d (overfed) of liquid HFD (37% kcal corn oil) by iG infusion for 45 d; 23 g/kg/d (low dose) or 32 g/kg/d (high dose) via iG infusion added to diet after 45; controls received isocaloric amount of diet; and (3) Duration: 10 wk | (1) ↑ hepatic TG, hepatic MDA, BAL in EtOH-treated overfed mice and (2) ↑ ALT in overfed mice; dose- dependent ↑ ALT by EtOH | (1) Steatosis, mononuclear cell infiltration in EtOH-treated (high dose) control mice; (2) Steatohepatitis and pericellular fibrosis in EtOH-treated (high dose) overfed mice; (3) ↑ macrophage infiltration in WAT in EtOH-treated overfed mice; and (4) No dif. in liver histology between EtOH-naïve overfed mice and controls | (1) ↑ M1 polarization markers (iNOS, TNFα) in liver and WAT for overfed-EtOH mice; (2) ↑ M2 markers (IL-10, ARG-1) in WAT, but ↓in liver for overfed- EtOH mice; (3) ↓ AdipoR, mitochondrial function genes (PGC-1α, PPARα, COX, cytochrome c, ACOX-1), in overfed-EtOH; (4) ↑ SREBP-1, ↓ pACC in EtOH; and (5) No effect on AMPK, PPARδ by overfeeding or EtOH | |
The most common type of dietary SMASH model couples HFD with either chronic or binge (acute) EtOH consumption. For example, Gäbele et al[77] gave Balb/c mice ad libitum access to either chow diet or HFD, along with water or 5% EtOH in drinking water. HFD-EtOH diet upregulated TNFα, TGFβ, collagen I, α smooth muscle actin (αSMA) expression, synergistically contributing to inflammation and fibrosis. HFD upregulated TLR-4 expression, but EtOH did not have an effect on TLR-4. However, combined HFD-EtOH increased serum LPS levels and had increased fibrosis compared to the other groups, suggesting that obesity increases LPS sensitivity by upregulating TLR-4, while EtOH promotes fibrosis by increasing intestinal permeability and LPS leakage.
Wang et al[78] used high-fat (HF) Lieber-DeCarli (LD) diet and observed increased inflammation and apoptosis in HF-EtOH treated rats. Sprague-Dawley rats were given ad libitum access to control LD or HF LD diet for 6 wk. The HF LD-fed rats were then given either HF LD and HF-EtOH LD for another 4 wk. HF-EtOH LD increased hepatic steatosis and number of inflammatory foci compared to controls. HF-EtOH LD also increased TUNEL+ apoptotic hepatocytes compared to control diet, but EtOH did not significantly affect TUNEL+ hepatocyte count, suggesting that obesogenic diet contributes more to apoptosis than EtOH in this model. There was no difference in B-cell lymphoma (BCL) 2 or BCL-2-associated X protein (Bax) between the groups, while Fas death receptor and Fas ligand were upregulated in the HF-EtOH LD group, suggesting that hepatocyte apoptosis occurs via the extrinsic pathway in SMASH.
Similarly, Gopal et al[79] developed a model using both solid diet and LD to determine the effect of exogenous SOD-1 on inflammation and fibrosis in SMASH. C57BL/6 mice were fed either chow or HFD for 10 wk, then either control LD or 5% EtOH LD for an additional 4 wk. The HFD-fed mice were also administered SOD-1 for 2 wk. HFD-EtOH upregulated CYP2E1, ADH, catalase, FA oxidizing ACOX-1, pro-inflammatory CCL-2, and pro-fibrotic MMP-12 compared to controls. These results corroborate the findings in the other aforementioned HFD-EtOH models that dietary obesity and EtOH both contribute to lipid dysregulation, inflammation, and fibrosis. SOD-1 treatment attenuated the increases in CYP2E1, ADH, CCL-2, and MMP-12, but further upregulated catalase, PPARα, and ACOX-1. The effects of SOD indicate that dysregulation of antioxidant mechanisms plays a role in SMASH pathogenesis, but targeting these mechanisms pharmacologically may have adverse effects on lipid metabolism (Figure 1).
The aforementioned models used chronic EtOH feeding to induce liver injury, but Duly et al[80] took a different approach to modeling SMASH by coupling HFD to repeated binge EtOH consumption. C57BL/6 mice were fed chow or HFD for 12 wk, and administered saline or 2 g EtOH/kg BW twice per week. ALT/AST activity did not differ between experimental groups, but HFD-EtOH increased hepatic TG levels compared to controls. HFD-EtOH also upregulated SREBP-1 and stearoyl-CoA desaturase 1, a transcriptional target of SREBP-1 involved in FA synthesis[16], compared to controls. HFD-EtOH also increased vimentin+ cells in the hepatic parenchyma and collagen deposition compared to controls, but did not affect expression of pro-fibrotic collagen I, TGFβ, and plasminogen activator inhibitor (PAI-1). These results suggest that obesity and EtOH synergistically contribute to lipid dysregulation and fibrosis, although through a yet unknown mechanism. The application of this study to human disease is also limited because HFD alone did not promote TG accumulation, a diagnostic criterion for NAFLD.
HFD intragastric infusion is also used in overfeeding models of SMASH. For example, Lazaro et al[81] fed C57BL/6 mice high saturated fat, high cholesterol (HFHC) diet for 2 wk, then liquid HFD or HFD-EtOH by intragastric infusion for 8 wk, with some HFD-EtOH-fed mice also receiving additional EtOH by gavage. HFHC and chronic-binge EtOH upregulated myeloperoxidase, CXCL-1, and OPN, and increased the presence of necrotic hepatocytes, suggesting increased hepatic neutrophil infiltration. HFHC-EtOH with additional EtOH binge also upregulated collagen I, αSMA, and TIMP-1 compared to controls. Unlike the Gäbele study, EtOH also upregulated TLR-4 expression in HFHC-fed mice. Since OPN is associated with increased ALD severity[82], HFHC-EtOH diet was also given to OPN-knockout (KO) mice to determine the role of OPN in liver disease. HFHC-EtOH diet increased neutrophil infiltration, plasma ALT activity, and αSMA in OPN KO mice, suggesting that OPN plays a protective role in fatty liver disease. Similarly, Xu et al[83] overfed C57BL/6 mice HFD by iG infusion for 45 d, after which different dosages of EtOH was introduced for another 4 wk. Compared to controls, overfeeding and EtOH elevated hepatic TG levels, increased fibrosis, upregulated induced nitric oxide synthase (iNOS) activity, and induced the PERK and IRE-1 pathways. HFD and high-dose EtOH increased liver weight, hepatic TG levels, plasma adiponectin levels, ALT activity, and fibrosis compared to low-dose EtOH treatment. These results suggest that concomitant overfeeding and EtOH consumption induce fibrosis, oxidative stress, and ER stress. Furthermore, these results indicate that the effects of EtOH on NAFLD progression are dose-dependent, as evidenced by the differential effects on liver injury by low and high dose EtOH.
To recapitulate SMASH pathogenesis, EtOH is also coupled with other dietary models of NAFLD other than HFD. These other dietary models of SMASH are similar to the HFD-based models in that they induce lipid dysregulation, oxidative stress, apoptosis, and fibrosis. Alwahsh et al[84] fed Sprague-Dawley rats either control LD, fructose LD, EtOH LD, or combined fructose-EtOH LD diet for 28 d. EtOH and fructose independently elevated hepatic TG and cholesterol, and upregulated PPARα and carnitine palmitoyltransferase I (CPT-1) compared to controls. However, combined fructose-EtOH decreased cholesterol, and downregulated PPARα and CPT-1 compared to controls. Combined treatment also upregulated leptin receptor and acetyl-CoA carboxylase (ACC). This study suggests that in either NAFLD or ALD, PPARα compensates for the increase in lipid accumulation, but FAO capacity is diminished under concomitant in SMASH. Nieto et al[85] developed a model in which Lewis rats were administered a choline-deficient (CD) diet and multiple whiskey gavages. Compared to controls, CD-whiskey treatment elevated MDA and protein carbonyl levels, upregulated TNFα, collagen I, αSMA, and β-tubulin, increased collagen deposition, and increased caspase-3 and caspase-8 activity. These results suggest that CD-whiskey induces oxidative stress, inflammation, and fibrosis. CD diet also downregulated BCL-XL, suggesting greater susceptibility to apoptosis, although whiskey did not affect its expression.
Experimental rodent models of SMASH also employ genetic models of NAFLD coupled with EtOH consumption. One commonly used genetic mouse model is ob/ob leptin deficiency. For example, Robin et al[86] developed a model in which ob/ob mice were administered EtOH daily by gavage for 4 d. EtOH upregulated TNFα and heat shock protein 70 (HSP70) expression, increased caspase-3 activity, and downregulated NF-κB expression and activity in obese mice compared to controls. Treatment with TNFα inhibitor pentoxifylline attenuated these effects in EtOH-treated obese mice. These results suggest that combined multiple binge EtOH dysregulates TNFα signaling and induces hepatocellular apoptosis in genetic obesity. However, EtOH did not increase serum ALT in either lean or obese mice, suggesting that it does not contribute to acute liver injury in this model.
Everitt et al[87] also used ob/ob mice to determine the effect of polyunsaturated fatty acids (PUFA) and chronic EtOH on obesity. EtOH increased ALT/AST, TG and cholesterol levels, and AMPK expression in obese mice compared to controls. EtOH also downregulated SIRT-1 and SFRS-10, a SIRT-1 target. SFRS-10 modulates splicing for the transcription factor lipin 1, which has two isoforms: FA oxidizing lipin 1α and TG synthetic lipin 1β[88]. The decrease in SFRS-10 resulted in an upregulation of the β isoform, which is also seen in human obesity and in vitro models[89]. In PUFA-fed ob/ob mice, EtOH also upregulated PPARγ which regulates adipocyte differentiation, glucose homeostasis, lipogenesis, and lipid droplet formation[90]. Although PPARγ is not upregulated in NASH patients[91], increased PPARγ and lipin-1β in the Everitt model underscores the potential synergistic effects of obesogenic factors and EtOH on AMPK-mediated de novo lipogenesis in human disease.
Another genetic mouse model is KK-Ay, which induces obesity and hyper-insulinemia by dysregulating appetite suppression. Suzuki et al[92] fed KK-Ay mice control or 5% EtOH LD diet for 10 d, then administered a single 4 g/kg EtOH dose by gavage in a chronic-binge EtOH model. In obese mice, EtOH upregulated spliced and unspliced XBP-1, BIP, inositol trisphosphate receptor, and C/EBP homologous protein, and oxidative stress markers CYP2E1 and heme oxygenase 1 (HMOX-1). Treatment with the chemical chaperone 4-phenylbutyric acid decreased the aforementioned UPR genes, ALT/AST activity, and hepatic TG levels. These results suggest that EtOH induces the UPR, exacerbates ER stress, and promotes oxidative stress in genetic obesity. In another study from the same lab, Kitagawa et al[93] used a similar feeding regiment and found elevated serum LPS, and upregulated inflammatory cytokines TNFα, IL-1β, and interferon γ, and upregulated TLR-4. Furthermore, with regards to the small intestinal microbiota, EtOH decreased Lactobacillales and increased Erysipelotrichales in the KK-Ay mice. Treatment with rifaximin, an antibiotic, restored the Erysipelotrichales population and increased Bacteroidales, while restoring cytokine expression to basal levels.
In rat studies, one commonly used genetic model is the fa/fa Zucker rat. For example, Carmiel-Haggai et al[94] administered fa/fa Zucker rats multiple binges of 35% EtOH or saline over 3 days to determine the effect of concomitant genetic obesity and EtOH consumption on oxidative stress. In obese fa/fa rats, EtOH decreased basal GSH levels, increased GSSG levels, and upregulated CYP2E1 and iNOS compared to controls. EtOH also decreased SOD, catalase, and GR activity in obese rats, suggesting that EtOH exacerbates oxidative stress in obesity by straining antioxidant mechanisms. Another genetic rat model is the Otsuka Long Evans Tokushima Fatty (OLETF) rat, which is a cholecystokinin KO model that dysregulates appetite suppression[95]. Minato et al[96] administered lean OLET and obese OLETF rats 10% EtOH gavage every few days for 3 wk in a multiple binge EtOH model of SMASH. In obese rats, EtOH increased inflammation, CYP2E1 protein levels, TNFα expression, 4-HNE levels compared to controls. However, EtOH did not affect PPARγ expression or steatosis in OLETF rats compared to EtOH-naïve OLETF rats. These results suggest that while this model recapitulates inflammation and oxidative stress in SMASH, it does not feature lipid metabolism dysregulation.
Some experimental models that couple obesity and EtOH consumption do not induce severe liver injury, and instead show evidence for a possible protective effect by moderate EtOH consumption in NAFLD. The amount of alcohol that constitutes “mild or moderate” (referred to as “moderate” hereafter) consumption is difficult to define, and there are varied definitions of moderate alcohol consumption in both humans and rodent models. In retrospective studies of NAFLD, moderate alcohol consumption is less than 20-40 g EtOH daily[97-99], although other studies define it as less than 2 alcoholic drinks per day[100]. Moderate alcohol consumption is associated with improved insulin sensitivity, decreased fibrosis, and improved cardiovascular outcomes in NAFLD/NASH patients[101-103], which is a significant finding because fibrosis is a major factor in NAFLD mortality[104]. However, the effects of BMI, insulin resistance, socioeconomic status, drinking pattern, or type of alcoholic beverage on human fatty liver disease have not been fully elucidated[103]. Nevertheless, there are rodent models that describe an apparent alleviation of NAFLD symptoms associated with moderate alcohol consumption. Moderate alcohol consumption is less rigorously defined for rodent models, although of the studies reviewed in this paper, those that model “moderate alcohol consumption” generally have less than a daily 5 g EtOH/kg body weight dosage for rodents[105-108].
Osaki et al[105] developed a model in which chronic consumption of 1%-2% v/v EtOH reduced blood markers for liver injury in HFD-fed Sprague-Dawley rats for 12 wk. EtOH did not affect body weight, liver mass, or adipose tissue mass, nor did it affect serum cholesterol, FFA, or TG levels. EtOH decreased serum ALT activity, lactate dehydrogenase, urate, and ammonia levels. In a similar study by Sun et al[107], a 4 g/kg EtOH binge by gavage downregulated expression of pro-inflammatory TNFα, CXCL-1, CXCL-2, and IL-1β, and pro- fibrotic αSMA, collagen, TGFβ, TIMP1, MMP2, and MMP9 in Sprague-Dawley rats fed HFHC for 12 wk. Using a genetic obesity model, Kanuri et al[106] determined the effect of chronic EtOH consumption on lipid storage and thrombosis. Ad libitum access to 2.5 g/kg EtOH was not found to affect TNFα, insulin receptor substrate (IRS)-1, or IRS-2 expression in ob/ob C57BL/6 mice. EtOH downregulated the expression of lipid droplet-associated proteins perilipin (PLIN) 2 and PLIN-3, suggesting decreased lipid accumulation due to EtOH. PLIN2 expression and lipid accumulation are known to be upregulated in ALD[109,110], but PLIN3 may instead play a protective role by facilitating lipid export from the ER[111]. Expression of pro-thrombotic PAI-1 was comparable between EtOH- treated ob/ob mice and control WT mice, despite upregulation of PAI-1 in EtOH-naïve ob/ob mice compared to WT.
Experimental animal studies have suggested the possible role of adiponectin signaling and sensitivity in apparent EtOH-mediated amelioration of NAFLD. For example, Bucher et al[112] found a decrease in serum adiponectin levels in C57BL/6 mice administered HFD and 10 g/kg/d EtOH in drinking water compared to mice fed HFD only. Decreased adiponectin in EtOH-treated mice accompanied lower serum TG and cholesterol levels, and downregulated collagen I, collagen III, and CYP2E1 expression. Similarly, Jung et al[108] found upregulated SIRT-1 and insulin receptor expression in high-fat, high-fructose, high-cholesterol-fed C57BL/6 mice administered pure EtOH or beer, compared to alcohol-naïve mice. Beer, but not pure EtOH, also upregulated adiponectin and adiponectin receptor 1 expression. In both of the aforementioned studies, AMPK activation by adiponectin suggests a possible compensatory effect in response to concomitant EtOH and lipid intake. However, the differential effects of beer and pure EtOH suggest that the alcohol-associated alleviation of fatty liver may instead be due to other components in alcoholic beverages. In an intervention study, serum adiponectin was elevated, although not significantly, in overweight (BMI ≥ 27 kg/m2) adults who consumed 100 mL whiskey daily for 4 wk, compared to overweight adults who were asked to drink mineral water[24].
Various pre-clinical experimental models have been developed to study the interactive effects of NAFLD and ALD risk factors on hepatic injury resulting in SMASH. While each model differs in obesity etiology, as well as pattern and duration of alcohol consumption, they all demonstrate that obesity and heavy alcohol consumption synergistically contribute to hepatic injury. The models suggest that both sets of risk factors contribute to increased steatosis and fibrosis, as well as an M1-like phenotype in activated macrophages. There is also evidence that obesity and alcohol consumption synergistically interfere with antioxidant defense, but the effect of alcohol seems to be dose-dependent. Despite the advantages of these animal models, there are still limitations with regards to replicating what we understand from human disease.
New models should consider the effect of alcohol consumption patterns on fatty liver disease pathogenesis. For example, the highest risk group for ALD are individuals who already drink heavily, then dramatically increase alcohol intake in a short period[113]. There are already models, such as the NIAAA model[75], that replicate chronic-binge alcohol consumption patterns in ALD. In the NIAAA model, mice are fed Lieber-DeCarli EtOH diet for 10 d and given EtOH gavage on the eleventh day, with a set of chronic-binge feedings that can repeated multiple times. However, repeated sets of chronic-binge feedings only slightly elevate ALT/AST levels compared to a single set, and does not result in liver fibrosis. Thus, future models should also induce advanced liver injury in the context of repeated chronic-binge alcohol consumption to fully recapitulate fatty liver disease in humans.
Furthermore, while most current experimental models use high-fat diets to induce obesity, future models should more closely replicate the diets that are composed of high-fat and high glycemic index foods that are commonly found in Western countries and have been identified as risk factors for NAFLD/NASH[114]. Fructose, in particular, is known to increase hepatic de novo lipogenesis and impair fatty acid oxidation in humans, and promote gut permeability in mice. Fructose also promotes insulin resistance in both the liver and skeletal muscle[115]. There are studies that model NAFLD progression that combine saturated fats, cholesterol, and fructose into a single diet[116,117], but the specific composition of fats and fructose source (e.g., sucrose or high fructose corn syrup) varies between studies. Future experimental models should thus also determine the effect of fructose on multi-system lipid dysregulation, insulin resistance, and inflammation.
Animal studies are also limited in replicating the genetic factors for NAFLD and ALD. For example, genetic models for obesity and insulin resistance in mice include ob/ob (leptin), db/db (leptin receptor), and foz/foz (ALMS-1)[118]. These do not parallel genetic polymorphisms known to predispose humans to NAFLD/NASH. Human genetic predispositions to fatty liver disease include polymorphisms in PNPLA3, TM6SF2, GCKR, MBOAT7, HSD17B3, PPARγ, IRS-1, PLIN2, and adiponectin, among others[119-121]. Furthermore, different rodent strains may also have an inherent bias towards certain disease states. For example, TNFα and iNOS are upregulated in C57BL/6 mice compared to Balb/c mice, despite being administered the same diet. Likewise, ARG-1 is increased in Balb/c compared to C57BL/6[122]. C57BL/6 mice may thus have a bias towards M1 polarization; and Balb/c, to M2 polarization. Future studies should further compare the roles that different human polymorphisms play in fatty liver disease pathogenesis, while also controlling for possible bias in the animal model used.
Finally, further research is needed to determine the interaction between multiple factors in fatty liver disease pathogenesis. In the established “two-hit” model of NAFLD pathogenesis, lipid accumulation results in a cascade of other dysfunctions, such as inflammation and fibrogenesis. Meanwhile, in the competing “multi-hit” model, multiple factors, such as insulin resistance and gut microbiome dysregulation, along with lipid metabolism dysregulation, synergistically result in liver injury[123]. For example, approximately 70% of all patients with insulin resistance will develop NAFLD. To determine possible interactions or causal relationships between these factors and alcohol, future experimental models should monitor fatty liver disease progression over time.
The development of novel pre-clinical experimental models may help elucidate new mechanisms and the interactions between alcohol and metabolic abnormalities in fatty liver disease. Specifically, feeding regimens that better model food and alcohol consumption in patients with SMASH should be developed. These models should incorporate both chronic-binge alcohol consumption and Western diet and also consider how those dietary factors interact with human genetics.
Manuscript source: Unsolicited Manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Association for the Study of Liver Diseases; Research Society on Alcoholism.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin H S-Editor: Zhang H L-Editor: A P-Editor: Liu JH
| 1. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1382] [Cited by in F6Publishing: 1772] [Article Influence: 354.4] [Reference Citation Analysis (0)] |
| 2. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1508] [Cited by in F6Publishing: 1595] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019;103:22-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 4. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1725] [Cited by in F6Publishing: 1672] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 5. | Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Åberg F, Puukka P, Salomaa V, Männistö S, Lundqvist A, Valsta L, Perola M, Jula A, Färkkilä M. Combined Effects of Alcohol and Metabolic Disorders in Patients With Chronic Liver Disease. Clin Gastroenterol Hepatol 2020; 18: 995-997. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Carr RM, Correnti J. Insulin resistance in clinical and experimental alcoholic liver disease. Ann N Y Acad Sci. 2015;1353:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 711] [Cited by in F6Publishing: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 13. | Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1847] [Cited by in F6Publishing: 2091] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 14. | You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730-E740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Jung EJ, Kwon SW, Jung BH, Oh SH, Lee BH. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J Lipid Res. 2011;52:1617-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, Takayanagi R, Nakamuta M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507-511. [PubMed] [Cited in This Article: ] |
| 18. | Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, Song BL. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011;13:44-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 793] [Cited by in F6Publishing: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 20. | Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 479] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 22. | You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892-G898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313-3327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 682] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 24. | Beulens JW, van Loon LJ, Kok FJ, Pelsers M, Bobbert T, Spranger J, Helander A, Hendriks HF. The effect of moderate alcohol consumption on adiponectin oligomers and muscle oxidative capacity: a human intervention study. Diabetologia. 2007;50:1388-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Bellanti F, Villani R, Facciorusso A, Vendemiale G, Serviddio G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic Biol Med. 2017;111:173-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Peter Guengerich F, Avadhani NG. Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens. Adv Exp Med Biol. 2018;1032:15-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Niemelä O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Han M, Matsumoto A, Wang Y, Thompson DC, Vasiliou V. Glutathione and Transsulfuration in Alcohol-Associated Tissue Injury and Carcinogenesis. Adv Exp Med Biol. 2018;1032:37-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Leclercq IA, Field J, Enriquez A, Farrell GC, Robertson GR. Constitutive and inducible expression of hepatic CYP2E1 in leptin-deficient ob/ob mice. Biochem Biophys Res Commun. 2000;268:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Mahli A, Thasler WE, Patsenker E, Müller S, Stickel F, Müller M, Seitz HK, Cederbaum AI, Hellerbrand C. Identification of cytochrome CYP2E1 as critical mediator of synergistic effects of alcohol and cellular lipid accumulation in hepatocytes in vitro. Oncotarget. 2015;6:41464-41478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Ozgur R, Uzilday B, Iwata Y, Koizumi N, Turkan I. Interplay between the unfolded protein response and reactive oxygen species: a dynamic duo. J Exp Bot. 2018;69:3333-3345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Liu X, Green RM. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019;3:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Henkel A, Green RM. The unfolded protein response in fatty liver disease. Semin Liver Dis. 2013;33:321-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Liu Z, Lv Y, Zhao N, Guan G, Wang J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015;6:e1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 374] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 37. | Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685-2700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 657] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 38. | He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44:532-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 987] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 39. | Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 688] [Cited by in F6Publishing: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 40. | Moreno-Sánchez R, Marín-Hernández Á, Gallardo-Pérez JC, Vázquez C, Rodríguez-Enríquez S, Saavedra E. Control of the NADPH supply and GSH recycling for oxidative stress management in hepatoma and liver mitochondria. Biochim Biophys Acta Bioenerg. 2018;1859:1138-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Świderska M, Maciejczyk M, Zalewska A, Pogorzelska J, Flisiak R, Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Free Radic Res. 2019;53:841-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Asghari S, Hamedi-Shahraki S, Amirkhizi F. Systemic redox imbalance in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2020;50:e13211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Chien YW, Chen YL, Peng HC, Hu JT, Yang SS, Yang SC. Impaired homocysteine metabolism in patients with alcoholic liver disease in Taiwan. Alcohol. 2016;54:33-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Szuster-Ciesielska A, Daniluk J, Kandefer-Szerszeń M. Oxidative stress in the blood of patients with alcohol-related liver cirrhosis. Med Sci Monit. 2002;8:CR419-CR424. [PubMed] [Cited in This Article: ] |
| 45. | Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;3:377-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 27] [Reference Citation Analysis (0)] |
| 46. | Byun JS, Yi HS. Hepatic Immune Microenvironment in Alcoholic and Nonalcoholic Liver Disease. Biomed Res Int. 2017;2017:6862439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 48. | Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 49. | Park JW, Jeong G, Kim SJ, Kim MK, Park SM. Predictors reflecting the pathological severity of non-alcoholic fatty liver disease: comprehensive study of clinical and immunohistochemical findings in younger Asian patients. J Gastroenterol Hepatol. 2007;22:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | De Vito R, Alisi A, Masotti A, Ceccarelli S, Panera N, Citti A, Salata M, Valenti L, Feldstein AE, Nobili V. Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med. 2012;30:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 512] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 52. | Hosseini N, Shor J, Szabo G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019;54:408-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 53. | Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 54. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1353] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 55. | Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3154] [Cited by in F6Publishing: 3333] [Article Influence: 208.3] [Reference Citation Analysis (0)] |
| 57. | Yang YM, Seki E. TNFα in liver fibrosis. Curr Pathobiol Rep. 2015;3:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 58. | Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 836] [Cited by in F6Publishing: 942] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 59. | Wallace MC, Friedman SL, Mann DA. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin Liver Dis. 2015;35:107-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 61. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1596] [Article Influence: 228.0] [Reference Citation Analysis (0)] |
| 62. | Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM Jr, Abumrad NN, Flynn CR. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270-G278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 63. | Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Yamada K, Mizukoshi E, Seike T, Horii R, Kitahara M, Sunagozaka H, Arai K, Yamashita T, Honda M, Kaneko S. Light alcohol consumption has the potential to suppress hepatocellular injury and liver fibrosis in non-alcoholic fatty liver disease. PLoS One. 2018;13:e0191026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Gandhi CR. Hepatic stellate cell activation and pro-fibrogenic signals. J Hepatol. 2017;67:1104-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Denk H, Abuja PM, Zatloukal K. Animal models of NAFLD from the pathologist's point of view. Biochim Biophys Acta Mol Basis Dis. 2019;1865:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Santhekadur PK, Kumar DP, Sanyal AJ. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 2018;68:230-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 68. | Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 69. | Sherriff JL, O'Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv Nutr. 2016;7:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 70. | Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol. 2017;241:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 71. | Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197-211. [PubMed] [Cited in This Article: ] |
| 72. | Tsukamoto H, French SW, Benson N, Delgado G, Rao GA, Larkin EC, Largman C. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5:224-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 186] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | French SW, Miyamoto K, Tsukamoto H. Ethanol-induced hepatic fibrosis in the rat: role of the amount of dietary fat. Alcohol Clin Exp Res. 1986;10:13S-19S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7:771-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8:627-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 698] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 76. | Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819-G823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Gäbele E, Dostert K, Dorn C, Patsenker E, Stickel F, Hellerbrand C. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcohol Clin Exp Res. 2011;35:1361-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Seitz HK, Wang XD. Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res. 2010;34:567-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Gopal T, Kumar N, Perriotte-Olson C, Casey CA, Donohue TM Jr, Harris EN, Talmon G, Kabanov AV, Saraswathi V. Nanoformulated SOD1 ameliorates the combined NASH and alcohol-associated liver disease partly via regulating CYP2E1 expression in adipose tissue and liver. Am J Physiol Gastrointest Liver Physiol. 2020;318:G428-G438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Duly AM, Alani B, Huang EY, Yee C, Haber PS, McLennan SV, Seth D. Effect of multiple binge alcohol on diet-induced liver injury in a mouse model of obesity. Nutr Diabetes. 2015;5:e154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, Xu J, Machida K, Tsukamoto H. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 82. | Seth D, Duly A, Kuo PC, McCaughan GW, Haber PS. Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activation. World J Gastroenterol. 2014;20:13088-13104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Xu J, Lai KKY, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Alwahsh SM, Xu M, Schultze FC, Wilting J, Mihm S, Raddatz D, Ramadori G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS One. 2014;9:e104220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Nieto N, Rojkind M. Repeated whiskey binges promote liver injury in rats fed a choline-deficient diet. J Hepatol. 2007;46:330-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor alpha and decreases nuclear factor-kappab to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005;42:1280-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Everitt H, Hu M, Ajmo JM, Rogers CQ, Liang X, Zhang R, Yin H, Choi A, Bennett ES, You M. Ethanol administration exacerbates the abnormalities in hepatic lipid oxidation in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G38-G47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Bi L, Jiang Z, Zhou J. The role of lipin-1 in the pathogenesis of alcoholic fatty liver. Alcohol Alcohol. 2015;50:146-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Pihlajamäki J, Lerin C, Itkonen P, Boes T, Floss T, Schroeder J, Dearie F, Crunkhorn S, Burak F, Jimenez-Chillaron JC, Kuulasmaa T, Miettinen P, Park PJ, Nasser I, Zhao Z, Zhang Z, Xu Y, Wurst W, Ren H, Morris AJ, Stamm S, Goldfine AB, Laakso M, Patti ME. Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell Metab. 2011;14:208-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 90. | Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 91. | Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 92. | Suzuki M, Kon K, Ikejima K, Arai K, Uchiyama A, Aoyama T, Yamashina S, Ueno T, Watanabe S. The Chemical Chaperone 4-Phenylbutyric Acid Prevents Alcohol-Induced Liver Injury in Obese KK-Ay Mice. Alcohol Clin Exp Res. 2019;43:617-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Kitagawa R, Kon K, Uchiyama A, Arai K, Yamashina S, Kuwahara-Arai K, Kirikae T, Ueno T, Ikejima K. Rifaximin prevents ethanol-induced liver injury in obese KK-Ay mice through modulation of small intestinal microbiota signature. Am J Physiol Gastrointest Liver Physiol. 2019;317:G707-G715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Carmiel-Haggai M, Cederbaum AI, Nieto N. Binge ethanol exposure increases liver injury in obese rats. Gastroenterology. 2003;125:1818-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav. 2008;94:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Minato T, Tsutsumi M, Tsuchishima M, Hayashi N, Saito T, Matsue Y, Toshikuni N, Arisawa T, George J. Binge alcohol consumption aggravates oxidative stress and promotes pathogenesis of NASH from obesity-induced simple steatosis. Mol Med. 2014;20:490-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Dunn W, Sanyal AJ, Brunt EM, Unalp-Arida A, Donohue M, McCullough AJ, Schwimmer JB. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol. 2012;57:384-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 98. | Cotrim HP, Freitas LA, Alves E, Almeida A, May DS, Caldwell S. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. Eur J Gastroenterol Hepatol. 2009;21:969-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Kwon HK, Greenson JK, Conjeevaram HS. Effect of lifetime alcohol consumption on the histological severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 100. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 883] [Article Influence: 63.1] [Reference Citation Analysis (1)] |
| 101. | Hsu CC, Kowdley KV. The Effects of Alcohol on Other Chronic Liver Diseases. Clin Liver Dis. 2016;20:581-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Ajmera VH, Terrault NA, Harrison SA. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? Hepatology. 2017;65:2090-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 103. | Weng G, Dunn W. Effect of alcohol consumption on nonalcoholic fatty liver disease. Transl Gastroenterol Hepatol. 2019;4:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Hagström H. Alcohol Consumption in Concomitant Liver Disease: How Much is Too Much? Curr Hepatol Rep. 2017;16:152-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Osaki A, Okazaki Y, Kimoto A, Izu H, Kato N. Beneficial effect of a low dose of ethanol on liver function and serum urate in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo). 2014;60:408-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Kanuri G, Landmann M, Priebs J, Spruss A, Löscher M, Ziegenhardt D, Röhl C, Degen C, Bergheim I. Moderate alcohol consumption diminishes the development of non-alcoholic fatty liver disease (NAFLD) in ob/ob mice. Eur J Nutr. 2016;55:1153-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Sun F, Zhuang Z, Zhang D, Chen Y, Liu S, Gao N, Shi J, Wang B. Chronic moderate alcohol consumption relieves high-fat high-cholesterol diet-induced liver fibrosis in a rat model. Clin Exp Pharmacol Physiol. 2018;45:1046-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Jung F, Lippmann T, Brandt A, Jin CJ, Engstler AJ, Baumann A. Moderate consumption of fermented alcoholic beverages diminishes diet-induced non-alcoholic fatty liver disease through mechanisms involving hepatic adiponectin signaling in mice. Eur J Nutr. 2020;59:787-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Carr RM, Dhir R, Yin X, Agarwal B, Ahima RS. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res. 2013;37:1091-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Carr RM, Peralta G, Yin X, Ahima RS. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One. 2014;9:e97118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 111. | Jeon S, Carr R. Alcohol effects on hepatic lipid metabolism. J Lipid Res. 2020;61:470-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 112. | Bucher S, Begriche K, Catheline D, Trak-Smayra V, Tiaho F, Coulouarn C, Pinon G, Lagadic-Gossmann D, Rioux V, Fromenty B. Moderate chronic ethanol consumption exerts beneficial effects on nonalcoholic fatty liver in mice fed a high-fat diet: possible role of higher formation of triglycerides enriched in monounsaturated fatty acids. Eur J Nutr. 2020;59:1619-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Choi G, Runyon BA. Alcoholic hepatitis: a clinician's guide. Clin Liver Dis. 2012;16:371-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Hosseini Z, Whiting SJ, Vatanparast H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr Res Rev. 2016;29:152-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 115. | Weber KS, Simon MC, Strassburger K, Markgraf DF, Buyken AE, Szendroedi J, Müssig K, Roden M; GDS Group. Habitual Fructose Intake Relates to Insulin Sensitivity and Fatty Liver Index in Recent-Onset Type 2 Diabetes Patients and Individuals without Diabetes. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Dorn C, Engelmann JC, Saugspier M, Koch A, Hartmann A, Müller M, Spang R, Bosserhoff A, Hellerbrand C. Increased expression of c-Jun in nonalcoholic fatty liver disease. Lab Invest. 2014;94:394-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 117. | Ganz M, Bukong TN, Csak T, Saha B, Park JK, Ambade A, Kodys K, Szabo G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J Transl Med. 2015;13:193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 118. | Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. Updates on Dietary Models of Nonalcoholic Fatty Liver Disease: Current Studies and Insights. Gene Expr. 2018;18:5-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 119. | Miyaaki H, Nakao K. Significance of genetic polymorphisms in patients with nonalcoholic fatty liver disease. Clin J Gastroenterol. 2017;10:201-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 121. | Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2020;17:40-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 122. | Maina V, Sutti S, Locatelli I, Vidali M, Mombello C, Bozzola C, Albano E. Bias in macrophage activation pattern influences non-alcoholic steatohepatitis (NASH) in mice. Clin Sci (Lond). 2012;122:545-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 123. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1741] [Article Influence: 217.6] [Reference Citation Analysis (1)] |
| 124. | Song M, Chen T, Prough RA, Cave MC, McClain CJ. Chronic alcohol consumption causes liver injury in high-fructose-fed male mice through enhanced hepatic inflammatory response. Alcohol Clin Exp Res. 2016;40:518-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |