Copyright
©The Author(s) 2020.
World J Gastroenterol. Nov 7, 2020; 26(41): 6346-6360
Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6346
Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6346
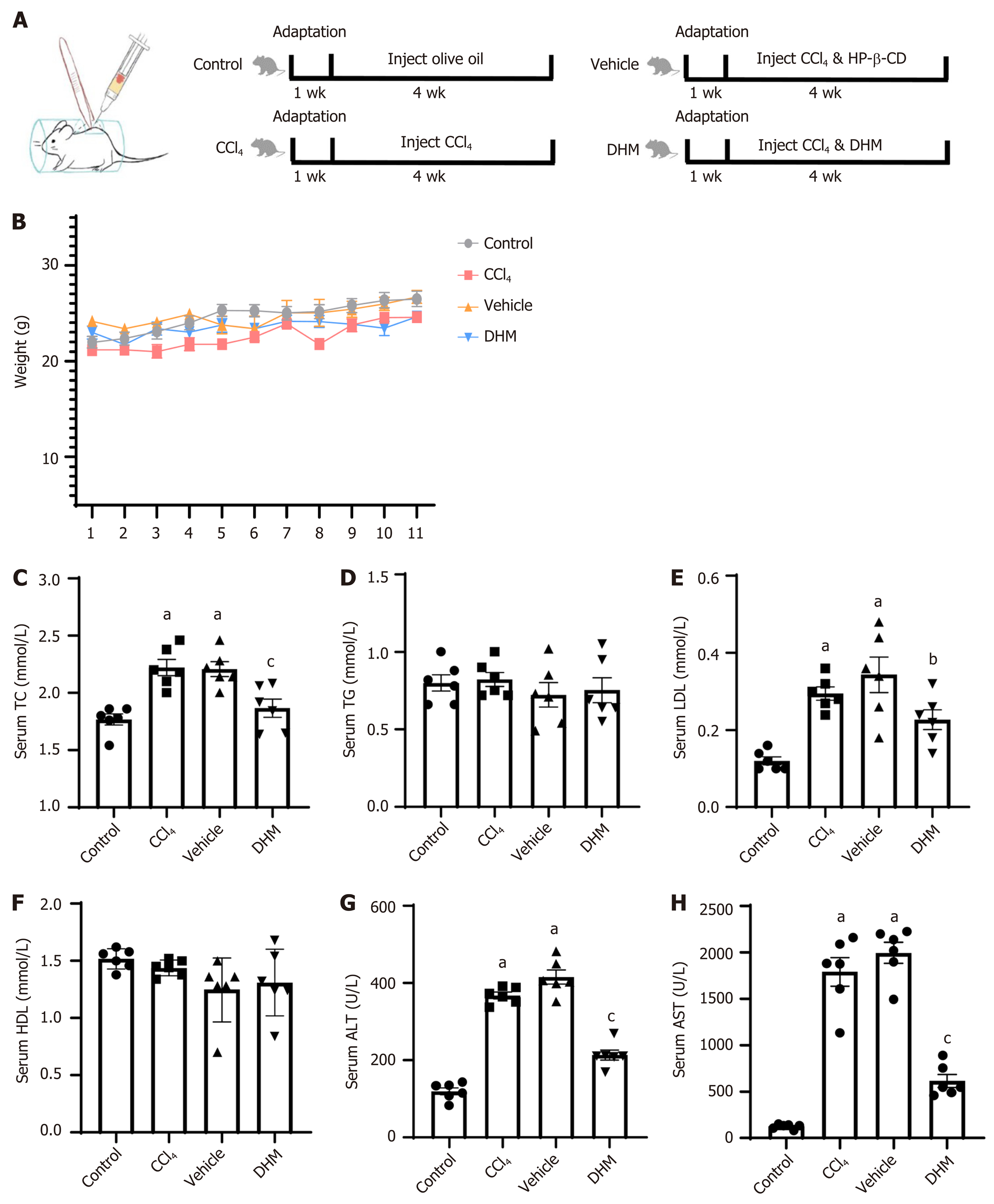
Figure 1 Modeling process, weight monitoring and serological testing.
A: Schematic diagram of subcutaneous injection into the back and modeling flow chart; B: Weight monitoring records; C-H: Statistical graphs of serum total cholesterol, triglyceride, low density lipoprotein, high density lipoprotein, alanine aminotransferase and aspartate aminotransferase, respectively. aP < 0.01 vs control group; bP < 0.05 vs vehicle group; cP < 0.01 vs vehicle group; n = 6. DHM: Dihydromyricetin; HP-β-CD: Hydroxypropyl-β-cyclodextrin; TC: Total cholesterol; TG: Triglyceride; LDL: Low density lipoprotein; HDL: High density lipoprotein; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
- Citation: Cheng QC, Fan J, Deng XW, Liu HC, Ding HR, Fang X, Wang JW, Chen CH, Zhang WG. Dihydromyricetin ameliorates chronic liver injury by reducing pyroptosis. World J Gastroenterol 2020; 26(41): 6346-6360
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6346